
Chemistry
10th Edition
ISBN: 9781305957404
Author: Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Question
Fill in the information based on the type of reaction mentioned in each column.
A. Draw the line angle diagram of a substrate that will undergo SN1, SN2, E1, E2
B. Draw the line angle diagram of a nucleophile that can be used for SN1, SN2, E1, and E2
C. Draw the line angle diagram of a solvent that can be used with SN1, SN2, E1, and E2
D. Write down if the stereochemistry of the product will be inversion, retention, or a 50:50 ratio of inversion and retention

Transcribed Image Text:SN1
SN2
E1
E2
Substrate (alkyl halide
example)
Formula of
nucleophile
Formula of Solvent
Streochemistry of
product – retention or
inversion or both
Expert Solution
arrow_forward
Step 1
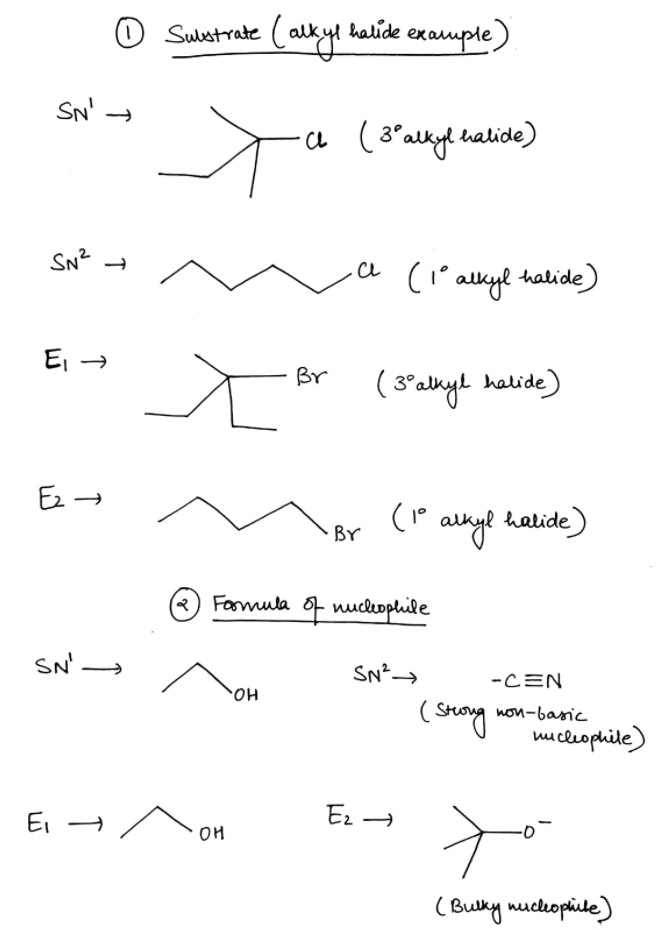
Trending nowThis is a popular solution!
Step by stepSolved in 2 steps with 2 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- In each reaction box, place the best reagent and conditions from the list below. There is more than one possible route. Note: İf one or more reagents are incorrectly placed, a single red X will appear on the top left. 1) Н, 2) 3) 4) 5) Br но 6) 1. CH3(CH2)2MgBr, 2. H2O 1. CH3CH2CH2CHO, 2. H2O PCC CH3(CH2)2OH 1. CH3MGB., 2. H2O CH3B Нзо* НОСH-CH2ОH, н* 1. CH3CHO, 2. Но Mgarrow_forwardQUESTION 3 Which answer correctly shows the second step of an E1 mechanism? A) EIÖH B) EIÖH C) EIÖH D), ELOH H. H H H H. H. H. D O O O Oarrow_forwardHelparrow_forward
- 5. (Rank the substrates in order of increasing reactivity in an SN2 reaction. (1 = least reactive, 4 = most reactive) Br of Br Br Brarrow_forwardQuestion 3 of 4 O Macmillan Learning Determine whether each of these reactions occur through an SN 1, SÃ2, E1, or E2 mechanism. A. > B. Br Br (CH3)3CONa (CH3)3COH CH3ONa THE O + Na Br + CH3 Na Br A. The mechanism of Reaction A is: SN2 E2 SN 1 E1 B. The mechanism of Reaction B is: E1 E2 SN2 SN 1arrow_forward1. Draw 2-methylpropanoic acid + thionyl chloride/pyridine catalysis + heat = ibuprofen intermediate C4H6OCI is the answer for the first intermediate (this one I already have it) 2. Draw ibuprofen intermediate 1 + benzene + AlCl3 + heat = ibuprofen intermediate 2. 3. Draw ibuprofen intermediate 2 + hydrazine = ibuprofen intermediate 3. 4. Draw ibuprofen intermediate 3 + KOH + heat = ibuprofen intermediate 4. 5. Draw ibuprofen intermediate 4 + ethanoyl chloride + AlCl3 + heat = ibuprofen intermediate 5. 6. Draw ibuprofen intermediate 5 + NaBH4 + hydronium workup = ibuprofen intermediate 6. (this one I have it already) 7. Draw ibuprofen intermediate 6 + HBr = ibuprofen intermediate 7. (this one i have it already)8. Draw ibuprofen intermediate 7 + Mg = ibuprofen intermediate 8. 9. Draw ibuprofen intermediate 8 + CO2 = ibuprofen intermediate 9. 10. this is the ibuprofen (i already have it)arrow_forward
- It will be helpful if I find the distinction between these two products since they are both Elimination reactions? Will one proceed via E1 or E2? If so, why is that the case?arrow_forward4) When a mixture of two different compounds both containing a hydrogens are placed under acidic or basic conditions, crossed aldol products can be formed. By either self-condensation or cross condensations. Draw the 4 aldol and 4 a.B-unsaturated ketones that can be made from the following mixed system. -OH 4 products One way to control this is by either having only one compound with a hydrogens or by reacting the desired nucleophile with lithium diisopropylamide (LDA) before addition of second carbonyl. The issue here is that this reaction stops at the aldol unless excess base is used. он LDA OH heat Show a synthetic scheme of how to use the following carbonyls to control the formation of each cross product (i.e. each as nucleophile and electrophile, two reactions). andarrow_forward11. Determine whether each reaction occurs by an SN2, SN1, E1, and/or E2 reaction. Provide a detailed mechanism with curved arrows and the major product(s). OMs Br Br OH мон OH NaSH DMF NaOH H₂O, heat LDA DMF NaOH H₂O, heat HBr H, H₂O heatarrow_forward
- 6. Provide the product for each of the following reaction. Indicate the type of reaction based on the reaction conditions. Show the complete mechanism for each reaction. Show the energy diagram for the reaction. F3C. CH3 CH3OH 25 °Carrow_forwardFor each problem a. Identify the nucleophile and the electrophile in each step.b. Depending on which is missing, either draw in the appropriate arrows toillustrate how the product is formed, or draw in the product of the reaction. c. If both are missing (aka 1e), both draw in the product and draw the appropriate arrows.arrow_forward7. Each of the following synthesis will not produce the desired product. Explain the flaw in each synthesis. NO2 1. HNO3, H2SO4. 2. ETCI, AICI, a. 1. Br2, FeBr3 2. AICI3, Br b.arrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY