
Introductory Chemistry For Today
8th Edition
ISBN: 9781285644561
Author: Seager
Publisher: Cengage
expand_more
expand_more
format_list_bulleted
Question
Draw the product to each of the following reactions.
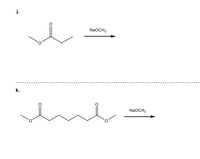
Transcribed Image Text:j.
NaOCH3
k.
NaOCH3
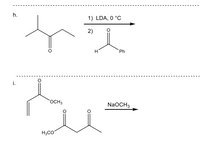
Transcribed Image Text:h.
1) LDA, 0 °C
2)
H
Ph
i.
OCH3
NaOCH3
H3CO
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution
Trending nowThis is a popular solution!
Step by stepSolved in 2 steps with 2 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- Question 17 Choose the situation below that would result in an exothermic AHsolution- When |AHsolventl JAHsolutel >> When JAHjatticel > JA Hnydrationl When JAHjatticel < JAHnydrationl When JAHlatticel is cole to |AHnydration There isn't enough information to determine.arrow_forward4). Which of the following is the best method for preparing aspirin? II III L COOH LOCCH3 COOH Oi OCCI HO-d-CH₂- + HCOOH + CH3Cl — COOH OCCH3 IV. V. ? COOH OH COOH OH + CHC−NH, _H $8-0-80 + CH3C-0-CCH3 HOarrow_forwardWhich of the following equilibrium equations have the smallest equilibrium constant? a) CH₂CH₂O`Na + -OH 0 CHOON b) (CH3)₂CHCO Na + H₂O c) NaOH + -OH CI d) More information is neededarrow_forward
- A substance that functions to prevent rapid, drastic changes in the pH of a body fluid by changing strong acids and bases into weak acids and bases is called an: a.salt. b.buffer. c.enzyme. d.coenzyme.arrow_forwardLactic acid, CH3CH(OH)COOH, is a weak monoprotic acid with a melting point of 53 C. It exists as two enantiomers (Sec. 7-2f) that have slightly different Ka values. The D form has a Ka of 1.5 104 and the L form has a Ka of 1.6 104. The D form is synthesized by some bacteria. The L form is produced in muscle cells during anaerobic metabolism in which glucose molecules are broken down into lactic acid and molecules of adenosine triphosphate (ATP) are formed. When lactic acid builds up too rapidly in muscle tissue, severe pain results. (a) Which form of lactic acid (D or L) is the stronger acid? Explain your answer. (b) Determine the pKa that would be measured for a 50:50 mixture of the two forms of lactic acid in aqueous solution, pKa = log Ka (c) A solution of D-lactic acid is prepared. Use HL as a general formula for lactic acid, and write the equation for the ionization of lactic acid in water. (d) If 0.100-M solutions of these two acids (D and L) were prepared, calculate what the pH of each solution would be. (e) Before any lactic acid dissolves in the water, what reaction determines the pH? (f) Calculate the pH of a solution made by dissolving 4.46 g D-lactic acid in 500. mL of water. (g) Calculate the volume (mL) of 1.15-M NaOH(aq) required to completely neutralize 4.46 g of pure lactic acid. (h) Calculate the pH of the solution when exactly enough NaOH was added to neutralize all of the lactic acid for (i) the D form; (ii) the L form; and (iii) a 50:50 mixture of the two forms.arrow_forwardThe labels on most pharmaceuticals state that the medicine should be stored in a cool, dark place. In the context of this chapter, explain why this is sound advice.arrow_forward
- If the G for a reaction is 4.5 kcal/mol at 298 K, what is the Keq for this reaction? What is the change in entropy of this reaction if H = 3.2 kcal/mol?arrow_forward1. A daily low dosage of aspirin (acetylsalicylic acid) can lower the risk of heart attack and stroke for individuals at risk. Aspirin suppresses the generation of prostaglandins by irreversible binding to the enzyme COX-1. Inhibition of prostaglandin synthesis lowers the production of thromboxanes that are responsible for the aggregation of platelets and the formation of blood clots. You will explore the mechanism of aspirin binding. 1.1 The average pH of the human body is 7.4 (also known as a physiological pH). Draw the structure of aspirin at a physiological pH. OH 1.2 Aspirin reacts with the amino acid Serine. What is the structure of serine? Draw the structure of the tripeptide Ala-Ser-Trp (draw these structures as they exist at physiological pH) serine Ala-Ser-Trp 1.3 Aspirin reacts with Serine 530 of COX-1 by transesterification reaction. Draw a general mechanism of acid-catalyzed transesterification reaction. Use HA as an acid. 1.4 Draw an arrow-pushing mechanism for the…arrow_forward1. LAH CO2Et 2. aq H*arrow_forward
- Is cyclopentanamine soluble in water? HCl? NaOH? NaHCO3?arrow_forwardPh O N O Me 1a. LDA, THF, -35 °C 1b. 2,3-dibromopropene 2a. LIAIH4, THF, -78 ° to 0 °C 2b. NaOH, H₂O workup E 66%arrow_forwardHyperphosphatemia may be found in O Hypoparathyroidism Rickets Hyperparathyroidism O Possible long-term use of aluminum hydroxide gel antacid O AOTA АОТАarrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 Chemistry for Today: General, Organic, and Bioche...ChemistryISBN:9781305960060Author:Spencer L. Seager, Michael R. Slabaugh, Maren S. HansenPublisher:Cengage Learning
Chemistry for Today: General, Organic, and Bioche...ChemistryISBN:9781305960060Author:Spencer L. Seager, Michael R. Slabaugh, Maren S. HansenPublisher:Cengage Learning Chemistry: The Molecular ScienceChemistryISBN:9781285199047Author:John W. Moore, Conrad L. StanitskiPublisher:Cengage Learning
Chemistry: The Molecular ScienceChemistryISBN:9781285199047Author:John W. Moore, Conrad L. StanitskiPublisher:Cengage Learning Chemistry for Engineering StudentsChemistryISBN:9781285199023Author:Lawrence S. Brown, Tom HolmePublisher:Cengage Learning
Chemistry for Engineering StudentsChemistryISBN:9781285199023Author:Lawrence S. Brown, Tom HolmePublisher:Cengage Learning Chemistry: Matter and ChangeChemistryISBN:9780078746376Author:Dinah Zike, Laurel Dingrando, Nicholas Hainen, Cheryl WistromPublisher:Glencoe/McGraw-Hill School Pub Co
Chemistry: Matter and ChangeChemistryISBN:9780078746376Author:Dinah Zike, Laurel Dingrando, Nicholas Hainen, Cheryl WistromPublisher:Glencoe/McGraw-Hill School Pub Co


Chemistry for Today: General, Organic, and Bioche...
Chemistry
ISBN:9781305960060
Author:Spencer L. Seager, Michael R. Slabaugh, Maren S. Hansen
Publisher:Cengage Learning

Chemistry: The Molecular Science
Chemistry
ISBN:9781285199047
Author:John W. Moore, Conrad L. Stanitski
Publisher:Cengage Learning

Chemistry for Engineering Students
Chemistry
ISBN:9781285199023
Author:Lawrence S. Brown, Tom Holme
Publisher:Cengage Learning


Chemistry: Matter and Change
Chemistry
ISBN:9780078746376
Author:Dinah Zike, Laurel Dingrando, Nicholas Hainen, Cheryl Wistrom
Publisher:Glencoe/McGraw-Hill School Pub Co