
Chemistry
10th Edition
ISBN: 9781305957404
Author: Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Concept explainers
Question
Name the compounds in the picture below.
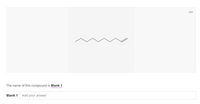
Transcribed Image Text:...
The name of this compound is Blank 1
Blank 1
Add your answer
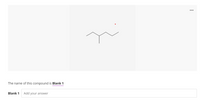
Transcribed Image Text:...
The name of this compound is Blank 1
Blank 1
Add your answer
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by stepSolved in 2 steps with 2 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- 27arrow_forwardWhat is the name of the compound with the formula Mg(NO3)2 ? fill in the blank 1What is the name of the compound with the formula BaSO4 ? fill in the blank 2What is the name of the compound with the formula CaCrO4 ?arrow_forwardWhat is the name of the compound with the formula BaI2?fill in the blank 1What is the name of the compound with the formula FePO4?fill in the blank 2What is the name of the compound with the formula CaSO3?fill in the blank 3arrow_forward
- Identify the missing information for each atom or ion. Note that the atoms and ions are not necessarily neutral. 1.) A SeSe ion has a mass number of 7777 and a charge of −2−2 . Determine the number of neutrons, protons, and electrons in this ion. 2.) An ion has a mass number of 65, 36 neutrons, and a charge of +1+1 . Identify the element symbol, and determine the number of protons and electrons in this ion. 3.) An atom or ion has 41 neutrons41 neutrons , 36 protons, and 36 electrons. Identify the element symbol, and determine the mass number and charge for this atom or ion.arrow_forwardName the compounds properlyarrow_forwardWhich of the elements below are not commonly found in living things? Group of answer choices oxygen lead sulfer carbonarrow_forward
- A compound is 32.88% C, 4.14% H, 19.18% N and 43.80% O by mass, and it has a molar mass of 219 g/mol. What is the molecular formula of this compound? When entering the formula place the elements in the following order: C, H, N, O. For subscripts, write the number after the element it corresponds to. For example, H2O should be written as H2O.arrow_forwardIdentify the following elements, giving their name and atomic symbol. a gaseous element in Group 7A, Period 3. a radioactive transition element in Period 5. the alkaline earth metal in Period 4. a metal that is normally a liquid.arrow_forwardfill in the missing name or formula for the compounds below.arrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY