
Organic Chemistry: A Guided Inquiry
2nd Edition
ISBN: 9780618974122
Author: Andrei Straumanis
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Concept explainers
Question
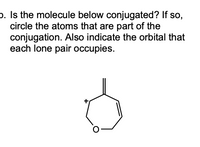
Transcribed Image Text:p. Is the molecule below conjugated? If so,
circle the atoms that are part of the
conjugation. Also indicate the orbital that
each lone pair occupies.
O-
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution
Trending nowThis is a popular solution!
Step by stepSolved in 3 steps with 1 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- Examine the ungraded ball-and-stick model below to determine the three-dimensional structure of the molecule. On the corresponding 2D structure, draw one wedge bond and one dash bond over two existing bonds to indicate the same arrangement of atoms in space. The narrow part of each wedge-and-dash bond should be towards the same central carbon atom.arrow_forward1. Which of the following molecules should have the longest max based on the idea it has the most continuous conjugation? A B C OH Darrow_forwarde. Draw the cis and trans isomer of the molecule above. f. Draw the chair form of the cis isomer of the molecule. g. Draw the flipped chair form of the cis isomer of the molecule. h. What is the more stable chair form?arrow_forward
- I had gotten this one incorrect, but i wanted to use it to study, so please give me the solution to all parts of this question.arrow_forwardCan you show me im trying to determine which portions are sp2 and sp3 so I can determine where ressonance occurs. Like label them so I can understand kind of the way I did, and then the Oxygen part I dont understand either. For instance I labeled a few but I want each C point and then the oxygen bc after doing so im going to use it to determine delocalized lone pairs and localized lone pairs.arrow_forwardClick on all of the atoms that make up the largest coplanar unit in the molecule below. XX H H -Harrow_forward
- See image belowarrow_forwardx-xo B Draw molecule A. On that drawing include the lone pairs and the curved arrows that would produce resonance structure B.arrow_forward\Br A. Redraw the bond-line structure of the molecule above on a separate sheet of paper. B. Draw a chair conformation for the molecule. C. Draw the new chair conformation after ring inversion (ring flip). D. Circle the most stable (lowest energy) chair conformation.arrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 Organic Chemistry: A Guided InquiryChemistryISBN:9780618974122Author:Andrei StraumanisPublisher:Cengage Learning
Organic Chemistry: A Guided InquiryChemistryISBN:9780618974122Author:Andrei StraumanisPublisher:Cengage Learning Chemistry for Today: General, Organic, and Bioche...ChemistryISBN:9781305960060Author:Spencer L. Seager, Michael R. Slabaugh, Maren S. HansenPublisher:Cengage Learning
Chemistry for Today: General, Organic, and Bioche...ChemistryISBN:9781305960060Author:Spencer L. Seager, Michael R. Slabaugh, Maren S. HansenPublisher:Cengage Learning

Organic Chemistry: A Guided Inquiry
Chemistry
ISBN:9780618974122
Author:Andrei Straumanis
Publisher:Cengage Learning


Chemistry for Today: General, Organic, and Bioche...
Chemistry
ISBN:9781305960060
Author:Spencer L. Seager, Michael R. Slabaugh, Maren S. Hansen
Publisher:Cengage Learning