
Chemistry
10th Edition
ISBN: 9781305957404
Author: Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Question
Transcribed Image Text:Moving to another question will save this response.
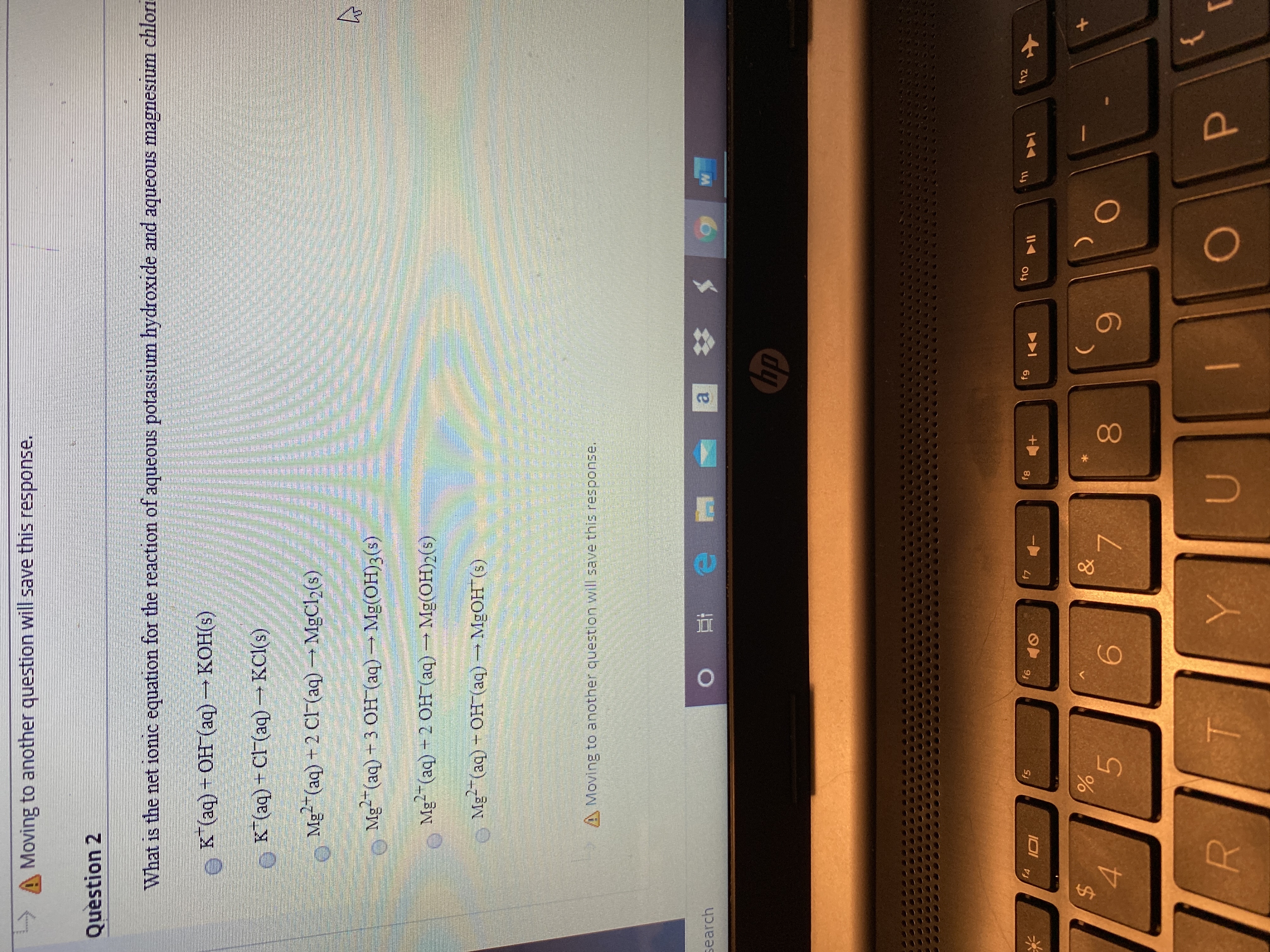
Question 2
What is the net ionic equation for the reaction of aqueous potassium hydroxide and aqueous magnesium chlori
1S
K (aq) + OH (aq) KOH(s)
КОНЕ)
K*(aq) + Cl (aq) →KCI(s)
Mg"(aq) + 2 CF (aq) MgCl2(s)
2+
o Mg-(aq)+3 OH (aq)Mg(0H)3(s)
Mg-(aq) -2 OH (aq) Mg(OH)2(s)
Me (aq) - Он (аq) — MgОН (9)
MGOH (s
A Moving to another question will save this response.
search
hp
f11
f12
fg
f10
f6
f7
f8
f4
f5
I미
&
%24
4.
6.
7.
80
T.
|
0O
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution
Trending nowThis is a popular solution!
Step by stepSolved in 2 steps with 3 images

Knowledge Booster
Similar questions
- Chlorine can be prepared in the laboratory by the reaction of manganese dioxide with hydrochloric acid, HCI(aq), as described by the chemical equation MnO, (s) + 4 HCI(aq) MnCl, (aq) + 2 H,0(1) + Cl, (g) How much MnO, (s) should be added to excess HCl(aq) to obtain 205 mL CI, (g) at 25 °C and 735 Torr? mass of MnO2: garrow_forwardChlorine can be prepared in the laboratory by the reaction of manganese dioxide with hydrochloric acid, HCl(aq), as described by the chemical equation MnO, (s) + 4 HCl(aq) - MnCl, (aq) + 2 H,O(1) + Cl, (g) How much Mn0, (s) should be added to excess HCI(aq) to obtain 235 mL Cl, (g) at 25 °C and 735 Torr? mass of MnO,: garrow_forwardOne way the U.S. Environmental Protection Agency (EPA) tests for chloride contaminants in water is by titrating a sample of silver nitrate solution. Any chloride anions in solution will combine with the silver cations to produce bright white silver chloride precipitate.Suppose an EPA chemist tests a 250.mL sample of groundwater known to be contaminated with cadmium chloride, which would react with silver nitrate solution like this: CdCl2(aq)+2AgNO3(aq)→2AgCl(s)+CdNO32(aq). The chemist adds 51.0mM silver nitrate solution to the sample until silver chloride stops forming. She then washes, dries, and weighs the precipitate. She finds she has collected 8.9mg of silver chloride. Calculate the concentration of cadmium chloride contaminant in the original groundwater sample. Be sure your answer has the correct number of significant digits.arrow_forward
- Some chemical reactants are listed in the table below. Complete the table by filling in the oxidation state of the highlighted atom. oxidation state of highlighted atom species Na (aq) H30"(aq) co? (aq) Cl,(9)arrow_forwardOne way in which the useful metal copper is produced is by dissolving the mineral azurite, which contains copper(II) carbonate, in concentrated sulfuric acid. The sulfuric acid reacts with the copper(II) carbonate to produce a blue solution of copper(II) sulfate. Scrap iron is then added to this solution, and pure copper metal precipitates out because of the following chemical reaction: Fe (s) + CuSO4 (aq) → Cu (s) + FeSO4 (aq)Suppose an industrial quality-control chemist analyzes a sample from a copper processing plant in the following way. He adds powdered iron to a 200.mL copper(II) sulfate sample from the plant until no more copper will precipitate. He then washes, dries, and weighs the precipitate, and finds that it has a mass of 73.mg . Calculate the original concentration of copper(II) sulfate in the sample. Be sure your answer has the correct number of significant digits.arrow_forwardOne way in which the useful metal copper is produced is by dissolving the mineral azurite, which contains copper(II) carbonate, in concentrated sulfuric acid. The sulfuric acid reacts with the copper(II) carbonate to produce a blue solution of copper(II) sulfate. Scrap iron is then added to this solution, and pure copper metal precipitates out because of the following chemical reaction: Fe(s) + CuSO4(aq) → Cu(s) + FeSO4 (aq) Suppose an industrial quality-control chemist analyzes a sample from a copper processing plant in the following way. He adds powdered iron to a 350. mL copper(II) sulfate sample from the plant until no more copper will precipitate. He then washes, dries, and weighs the precipitate, and finds that it has a mass of 111. mg. Calculate the original concentration of copper(II) sulfate in the sample. Be sure your answer has the correct number of significant digits. 0-2 X 5arrow_forward
- Write the net ionic equation, including phases, that corresponds to the reaction Cu(NO3)2(aq)+K2S(aq)⟶CuS(s)+2KNO3(aq)arrow_forwardOne way in which the useful metal copper is produced is by dissolving the mineral azurite, which contains copper(II) carbonate, in concentrated sulfuric acid. The sulfuric acid reacts with the copper(II) carbonate to produce a blue solution of copper(II) sulfate. Scrap iron is then added to this solution, and pure copper metal precipitates out because of the following chemical reaction: Fe (s) + CuSO4 (aq) → Cu (s) + FeSO4 (aq)Suppose an industrial quality-control chemist analyzes a sample from a copper processing plant in the following way. He adds powdered iron to a 300.mL copper(II) sulfate sample from the plant until no more copper will precipitate. He then washes, dries, and weighs the precipitate, and finds that it has a mass of 67.mg . Calculate the original concentration of copper(II) sulfate in the sample. Be sure your answer has the correct number of significant digits.arrow_forwardChlorine can be prepared in the laboratory by the reaction of manganese dioxide with hydrochloric acid, HCl(aq), as described by the chemical equation MnO, (s) + 4 HCI(aq) – MnCl, (aq) + 2 H,0(1) + Cl, (g) > How much MnO, (s) should be added to excess HCl(aq) to obtain 105 mL Cl, (g) at 25 °C and 755 Torr? mass of MnO,:arrow_forward
- When an ionic solid dissolves in water, water molecules attract the ions causing them to dissociate orcome apart. The resulting dissolved ions are electrically charged particles that allow the solution toconduct electricity. The following chemical equations represent this phenomenon:NaCl (s) → Na+ (aq) + Cl-(aq)Na2CO3 (s) → 2Na+(aq) + CO32- (aq)Write a similar balanced chemical equation for the other four strong electrolytes in the data table NaHCO3 KNO3NH4Cl HClarrow_forward[References] Hydrogen cyanide, HCN, is prepared from ammonia, air, and natural gas ( CH4) by the following process: 2NH3 (g) + 30,(g) + 2CH, (g) →→ 2HCN(g) + 6H,0(g) Hydrogen cyanide is used to prepare sodium cyanide, which is used in part to obtain gold from gold-containing rock. If a reaction vessel contains 5.75 g NH3, 12.0 g 02, and 5.00 g CH4, what is the maximum mass in grams of hydrogen cyanide that could be made, assuming the reaction goes to completion as written? Mass = HCNarrow_forwarda For the reaction СаBra (aq) + K,SO4 (ag) write the balanced formula equation. (Be sure to specify states such as (aq) or (s). If a box is not needed, leave it blank. If no reaction occurs, leave all boxes blank and click on Submit.) СаBr, (aq) K,SO,(aq) CaSO,(s) | 2KC1(aq)arrow_forward
arrow_back_ios
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY