
Chemistry
10th Edition
ISBN: 9781305957404
Author: Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Question
Transcribed Image Text:E.
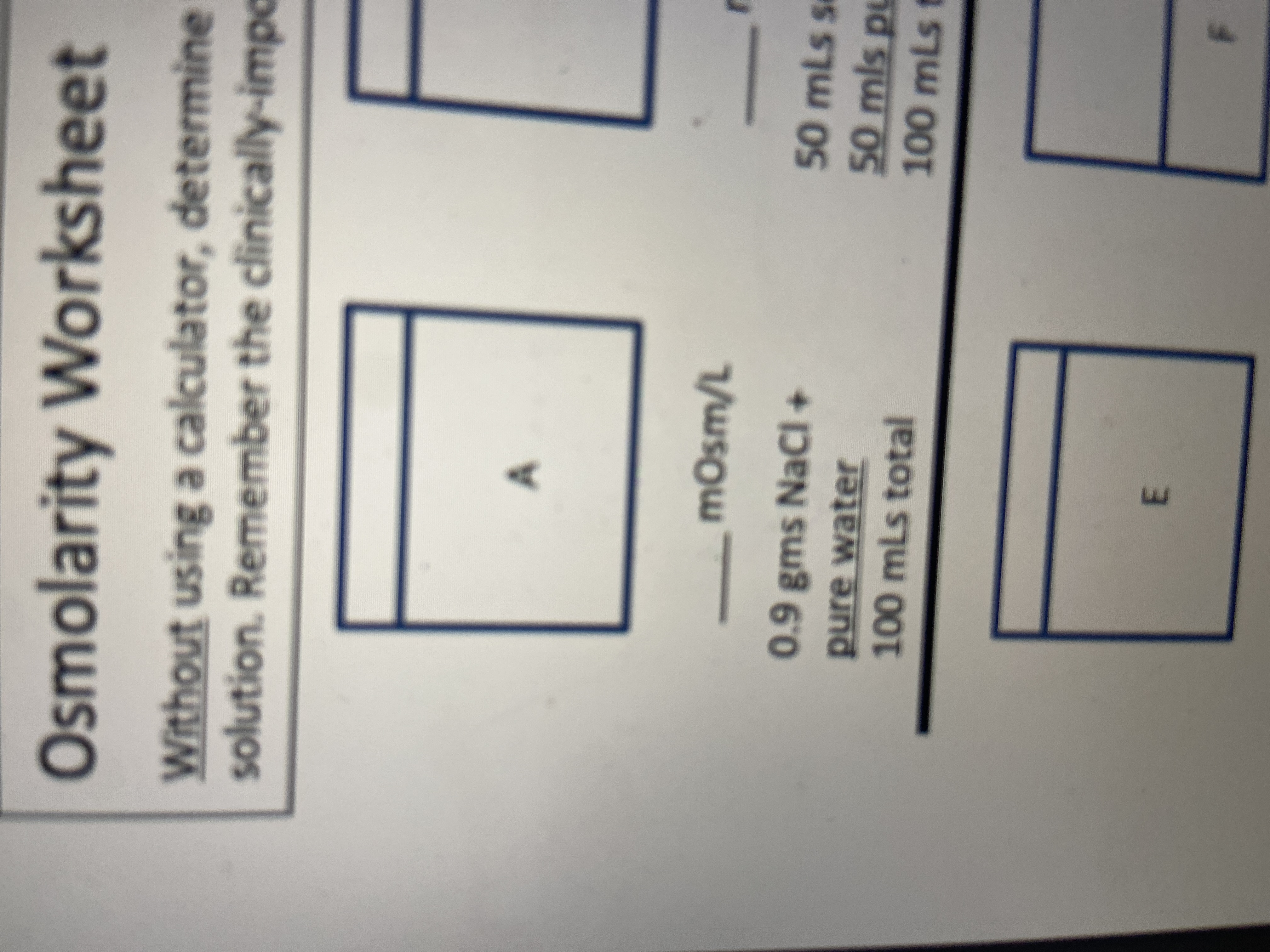
Osmolarity Worksheet
Without using a calculator, determine
solution. Remember the clinically-impc
mOsm/L
0.9 gms NaCl +
50 mLs s
pure water
100mLs total
50 mls pu
100mLs t
Expert Solution
arrow_forward
Step 1
Mass of NaCl = 0.9g
Mass of solution = 100mL
Molar mass of NaCl = 58g/mol
Trending nowThis is a popular solution!
Step by stepSolved in 2 steps

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- Consider the reaction of a 20.0 mL of 0.220 M C5H5NHCI (Ka = 5.9 x 10-6) with 12.0 mL of 0.217 M CSOH. Write the net ionic equation for the reaction that takes place. Be sure to include the proper phases for all species within the reaction.arrow_forwardSTATES OF MATTER Applying like dissolves like HO- solute : 0: || HOC-CH- OH I I OH OH I -CH₂-CH-CH-CH-CH-C-H : 0: || CH₂-C-OH | OH : 0: || NH,−C–NH, I OH : 0: || H H Which is the better solvent? :0: || CH₂ C- H 1 H-C CH₂OH Q2 CH, OH H-C .C II H H H 1 - CH₂ CH₂(CH₂), CH₂ CIH H H | C-H H : 0: || C-C-OH H 3/5arrow_forward1. If Mg(OH)2 has a Ksp of 7.0 × 10–6, then what is the solubility of Mg(OH)2 in pure water? Give your answer in units of g/L. Express the answer to 4 significant digits and do not use scientific notation. *Use the Ksp given here. Do not look up your own value. 2. 200.0 mL of 0.005 M Na2CO3 are added to 200.0 mL of 0.002 M MgCl2. Does a precipitate form? [Ksp(MgCO3) = 6.82 × 10–6] Question 2 options: No, because Q = Ksp. Yes, because Q > Ksp. No, because Q < Ksp. No, because Q > Ksp. Yes, because Q < Ksp. 3.In order for a precipitate of PbBr2 to form, what is the minimum concentration of Br- that must be present in a solution of 0.00050 M Pb(NO3)2. The Ksp of PbBr2 is 2.20 × 10–6.arrow_forward
- Pls help ASAParrow_forwardWhich substance is more soluble? CaSO4 (Ksp=7.10x10-5) AgCI(Ksp 1.77x10-10) O CaSO4 O AgClarrow_forwardGases, Liquids, and Solids Applying like dissolves like For each solute, click the button under the better solvent. H H solute Which is the better solvent? CC₁₁ CaCl2 CH₂OH 0 H C H H H₂O H 10: NH,C — NH, H H CH3(CH2)CH3 O CH, (CH₂),CH, H :0: || C-C-OH H X H 13arrow_forward
- 7arrow_forwardIf the solubility of a compound is 0.62 g in 100 mL of H2O at 0oC and 5.18 g in 100 mL of H2O at 100oC, what is the percentage of the compound recovered in recrystallization? Round your answer to 1 decimal place.arrow_forwardtGPT translate Google Search X C Which of the statements bel X oads/att.epCqDCR-ol0GROgrwWy0F2UVDmra3oKTRAHPREHOKQ.pdf OKTRAHPREHOKQ.pdf 20/60 150%+ 15. Njehsoni pH e një tretësire 0,010 M CH3CO2H. Ka = 1,8 x 10-5 për acidin acetik. A) 9.26 B) 3.39 C) 2.02 D) 10.61 E) 4.74 Përgjigje: B 16. Njehsoni fortësinë jonike të një tretësire 0.210 M FeCl2. Përgjigja: 0.630arrow_forward
- To prepare 200.0 mL of 0.01 M EDTA solution, how many grams of EDTA disodium salt dihydrate (C10H14N2Na2O8 · 2H2O) do you need?arrow_forwardName: Date: Section: Report Sheet: Determination of Molecular Weight Through Freezing Point Depression Analysis 30 Part A: Volume of Cyclohexane mL Mass of cyclohexane (d =0.7786 g/mL) 6.0 °C Freezing Point of cyclohexane 128 Part B: Molecular Weight of Naphthalene g/mol 0.23 Mass of Sample 1 Moles of Sample 1 Mass of Sample 2 0.18 Moles of Sample 2 Moles m (mol/kg solvent) T,('C) AT, Naphthalene 5.1 TRIAL 1 5.3 TRAIL 2 k, Cyclohexane CC/m) Part C: Unknown Number 0.21 0.19 Mass of Sample 1 Mass of Sample 2 Grams Unknown T,('C) AT 0.21 4.8 TRIAL 1 0.19 5.0 TRAIL 2 Moles UnknownTRIAL 1 (moles) TRIAL 2 (moles) TRIAL 1 (g/mol)TRIAL 2 (g/mol) Molecular Weight Unknown (g/mol) AVERAGE MOLECULAR WEIGHT OF UNKNOWNarrow_forwardSiven that the solubility of NaCl is 35.7 g/100 mL solution, calculate the 'accepted" value of the Ksp of NaCl.arrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY