
Chemistry
10th Edition
ISBN: 9781305957404
Author: Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Question
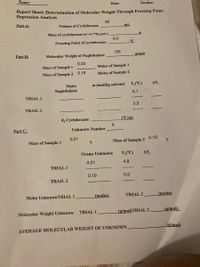
Transcribed Image Text:Name:
Date:
Section:
Report Sheet: Determination of Molecular Weight Through Freezing Point
Depression Analysis
30
Part A:
Volume of Cyclohexane
mL
Mass of cyclohexane (d =0.7786 g/mL)
6.0
°C
Freezing Point of cyclohexane
128
Part B:
Molecular Weight of Naphthalene
g/mol
0.23
Mass of Sample 1
Moles of Sample 1
Mass of Sample 2
0.18
Moles of Sample 2
Moles
m (mol/kg solvent) T,('C)
AT,
Naphthalene
5.1
TRIAL 1
5.3
TRAIL 2
k, Cyclohexane
CC/m)
Part C:
Unknown Number
0.21
0.19
Mass of Sample 1
Mass of Sample 2
Grams Unknown
T,('C)
AT
0.21
4.8
TRIAL 1
0.19
5.0
TRAIL 2
Moles UnknownTRIAL 1
(moles)
TRIAL 2
(moles)
TRIAL 1
(g/mol)TRIAL 2
(g/mol)
Molecular Weight Unknown
(g/mol)
AVERAGE MOLECULAR WEIGHT OF UNKNOWN
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by stepSolved in 5 steps with 5 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- Which of the following would affect the freezing point of water the most? A. Concentrated CaCl2 B. Concentrated NaCl C.  dilute NaCl D. dilute CaCl2arrow_forwardA student determines the molar mass of methanol, CH3OH by the same method used in this experiment. She found that the equilibrium temperature of ice and pure water was 0.4 ˚C. When she added 10.0 g of her sample (CH3OH), it fell to -5.4˚C. The mass of the solution was 101.8 g. What was the freezing point depression? What was the molality of CH3OH? How many moles of CH3OH are in the solution? Find the molar mass of CH3OH from the experimental data. How does it compare the value calculated from the periodic table?arrow_forwardGiven the following mixture of two compounds 65.00 mL of X (MW =82.00 g/mol)(density 0.808 g/mL) and 725.00 mL of Y (74.00 g/mol))(density 0.842 g/mL). The freezing point of pure Y is 27 00 degrees C. The molal freezing constant is 3.493 degrees C/m. What is the freezing point of the solution.arrow_forward
- Solution freezing pointarrow_forward3) Calculate the freezing point (oC) of a solution of 15.46 g of acetone (CH3COCH3, MM = 58.08 g/mol) in 85.0 g of CCl4, whose normal freezing point is –23.0 oC. Given: P = 1 atmKf CCl4 = 29.8 oC/m a) –55.6 b) –93.3 c) 35.7 d) –76.4 e) 70.3 f) –116arrow_forwardGiven the following mixture of two compounds 70.00 mL of X (MW =80.00 g/mol)(density 0.800 g/mL) and 820.00 mL of Y (73.00 g/mol))(density 1.056 g/mL). The freezing point of pure Y is 17.00 degrees C. The molal freezing constant is 3.091 degrees C/m. What is the freezing point of the solution, in degrees C?arrow_forward
- In an aqueous solution of sulfuric acid, the concentration is 7.21 mol% of acid. The density of the solution is 1.2033 g mL-1. Calculate the molal concentration of the H2SO4. Molality = i m H2SO4 Calculate the mass percent of the acid. % (w/w) H2SO4 Calculate the molarity of the solution. Molarity = MH2SO4arrow_forwardMoving to another question Wll tion 10 Calculate the molality of C2H5OH (molar mass = 46.068 g mol-1) in a water solution that is prepared by mixing 40.17 g of C2H5OH with 441.6 mL of H2U at 20 °C. (Density of water 1.00 g mL) (the answer should be entered with 3 significant figures; do not enter units; give answer in normal notation--examples include 1.23 and 120. and -123 and 123. and 12.3) K Question 10 of 29 A Moving to another question will save this response. 211 * LO, %23 24 & 2. 3 8 delete 4 E R Y [O P ente S D F G K L. ret C- V alt alt option n command command optionarrow_forwardA bottle on the stockroom shelf reads 41% sodium hydroxide solution. What is the molality of this solution? Recall that the % means (w/w) percentage. molality = m NaOH eTextbook and Media Save for Later Attempts: 0 of 15 used Submit A O S MacBook Airarrow_forward
- What is the molality of a4.60%v/vsolution of isopropanol. The density of water is0.9978 g/mLand the density of isopropanol is0.786 g/mL. The molar mass of water is18.02 g/moland the molecular mass of isopropanol is60.1 g/mol. ( please type answer not write by hend )arrow_forward10:16 S 57% Question 11 of 15 0/2 POINTS EARNED A solution made by dissolving 150.0 g of an unknown compound in 425.0 mL of 12 benzene to make a solution which has a freezing point 18.6 °C lower than that of pure benzene. Kf for benzene is 5.12 °C/m and the density of benzene is 0.877 g/mL. What was the molality of the solution? a O of 0.67 points earned L 3 attempts remaining How many moles of the unknown compound were dissolved in the b solution? L 0 of 0.67 points earned 3 attempts remaining What is the molar mass of the unknown compound? L O of 0.67 points earned 3 attempts remaining IIarrow_forwardPlease find the following with the information provided in the pictures: -freezing point of solvent - freezing point of solution - depression of freezing point - molecular weight of the unknown - determine molality - determine moles of solute - determine molecular weight weight of solvent is: 25.004g weight of solute is: 4.502garrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY