
Chemistry
10th Edition
ISBN: 9781305957404
Author: Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Question
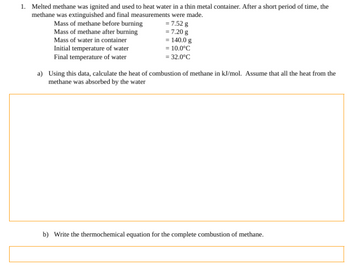
Transcribed Image Text:1. Melted methane was ignited and used to heat water in a thin metal container. After a short period of time, the
methane was extinguished and final measurements were made.
= 7.52 g
= 7.20 g
Mass of methane before burning
Mass of methane after burning
Mass of water in container
Initial temperature of water
Final temperature of water
= 140.0 g
= 10.0°C
= 32.0°C
a) Using this data, calculate the heat of combustion of methane in kJ/mol. Assume that all the heat from the
methane was absorbed by the water
b) Write the thermochemical equation for the complete combustion of methane.
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by stepSolved in 4 steps with 2 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- A chemical reaction takes place inside a flask submerged in a water bath. The water bath contains 2.30 kg of water at 35.8 °C. During the reaction 74.9 kJ of heat flows out of the bath and into the flask. Calculate the new temperature of the water bath. You can assume the specific heat capacity of water under these conditions 1 is 4.18 J.g •K`1 ▪ Be sure your answer has the correct number of significant digits.arrow_forwardA coffee cup calorimeter, including that water it contains, has a heat capacity of 425 J/K, and is initially at a temperature of 23.07 C. A 16.9 gram piece of nickel metal, initially at a temperature of 4.0°C is placed in the calorimeter. The final temperature of the calorimeter and the metal is 22.74°C. What is the specific heat of nickel metal (in Jg1 K-1)? 27 O0.44 1.4 O 25 O 145arrow_forwardA chemist carefully measures the amount of heat needed to raise the temperature of a 1.54 kg sample of C5H₁2S from 5.8 °C to 17.4 °C. The experiment shows that 3.51 × 104 J of heat are needed. What can the chemist report for the molar heat capacity of C5H₁2S? Be sure your answer has the correct number of significant digits. 12 - 1 – 1 K J. mol x10 X Ś 6 0 Uarrow_forward
- Use the References to access important values if needed for this question Ethanol, C2H0, is most often blended with gasoline - usually as a 10 percent mix - to create a fuel called gasohol. Ethanol is a renewable resource and ethanol-blended fuels, like gasohol, appear to bum more efficiently in internal combustion engines. The combustion enthalpy of ethanol is 1366.9 kJ/mol The combustion enthalpy of 2-methylpentane, C6H14 is 4.157x103 kJ/mol. Calculate the energy released during the complete combustion of 382 g 2-methylpentane kJ Assuming the same efficiency, would 382 g ethanol provide more, less, or the same quantity of energy as 382 g 2-methylpentane? | Submit Answer Retry Entire Group 8 more group attempts remainingarrow_forwardCalculate the energy required to heat 403.0 mg of water from 36.8 °C to 57.0 °C. Assume the specific heat capacity of water under these conditions is 4.18 J-g .K . Be sure your answer has the correct number of significant digits. 0 0x10 ロ・ロ X μ 00 4 Sarrow_forwardA chemist carefully measures the amount of heat needed to raise the temperature of a 399.0 g sample of a pure substance from 4.1 °C to 19.7°C. The experiment shows that 865. J of heat are needed. What can the chemist report for the specific heat capacity of the substance? Round your answer to 3 significant digits. -1 -1 J.g •K x10 Submit Assignme Continue 2022 McGraw Hill LLC. All Rights Reserved. Terms of Use Privacy Center | Accessibili I IMG-6111.jpg IMG-6110.jpg IMG-6109.jpg IMG-6108.jpg IMG-6107.jpg MacBook Air 80 DII DD F2 F3 F4 F5 F6 F7 F8 F9 F10 F11 @ # $ % & 2 3 4 5 6 7 8 9arrow_forward
- Review | Constants | Periodic Table To determine whether a shiny gold-colored rock is actually gold, a chemistry student decides to measure its heat çapacity. She first weighs the rock and finds it has a mass of 4.7 g. She then finds that upon absorption of 52.7 J of heat, the temperature of the rock rises from 25 °C to 57 °C. Find the specific heat capacity of the substance composing the rock. gh Expréss the specific heat capacity in joules per gram-Celsius to two decimal places. ? J Cs = g.°C Submit Request Answer Part B Complete previous part(s) Part C A 55.0 g aluminum block initially at 27.5 °C absorbs 725 J of heat. What is the final temperature of the aluminum? Express your answer in degrees Celsius to one decimal place. VO AEO T = P Pearson °C . All rights reserved. Terms of Use | Privacy Policy Permissions Contact Us |arrow_forwardCalculate the energy required to heat 718.0 mg of water from 46.0 °C to 56.7 °C. Assume the specific heat capacity of water under these conditions is -1 -1 4.18 J∙g¯¹·K-¹ Be sure your answer has the correct number of significant digits. 0 x10 X μ 010 ? 00. 18 Ararrow_forwardA 28.5 g piece of gold is heated and then allowed to cool. What is the change in temperature (°C) if the gold releases 0.226 kJ of heat as it cools? The molar heat capacity of gold is 25.4 J/mol・°C.arrow_forward
- A chemist carefully measures the amount of heat needed to raise the temperature of a 0.57 kg sample of a pure substance from 18.0 °C to 35.9 °C. The experiment shows that 19. kJ of heat are needed. What can the chemist report for the specific heat capacity of the substance? Round your answer to 2 significant digits. - 1 - 1 •K J. g x10arrow_forwardCan you explain where did I go wrong, I keep getting the same answer.arrow_forward
arrow_back_ios
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY