
Introductory Chemistry For Today
8th Edition
ISBN: 9781285644561
Author: Seager
Publisher: Cengage
expand_more
expand_more
format_list_bulleted
Question
help please answer in text form with proper workings and explanation for each and every part and steps with concept and introduction no AI no copy paste remember answer must be in proper format with all working
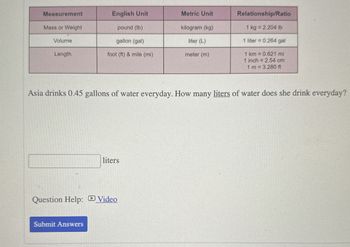
Transcribed Image Text:Measurement
English Unit
Metric Unit
Relationship/Ratio
Mass or Weight
Volume
pound (lb)
gallon (gal)
kilogram (kg)
liter (L)
1 kg = 2.204 lb
1 liter = 0.264 gal
Length
foot (ft) & mile (mi)
meter (m)
1 km
0.621 mi
1 inch = 2.54 cm
1 m = 3.280 ft
Asia drinks 0.45 gallons of water everyday. How many liters of water does she drink everyday?
liters
Question Help: Video
Submit Answers
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by stepSolved in 2 steps

Knowledge Booster
Similar questions
- Given that one metric ton = 1000 kg, how many metric tons are inlb?arrow_forwardAlthough the preferred SI unit of area is the square meter, land is often measured in the metric system in hectares (ha). One hectare is equal to 10,000 m2. In the English system, land is often measured in acres (1 acre = 160 rod2). Use the exact conversions and those given in Exercise 47 to calculate the following. a. 1 ha = __________ km2 b. The area of a 5.5-acre plot of land in hectares, square meters, and square kilometers c. A lot with dimensions 120 ft by 75 ft is to be sold for 6500. What is the price per acre? What is the price per hectare?arrow_forwardEJIYZPROfspoHcK3uA&lv=HbAhnaEn7foZmyeo a hjkml,/t[plkojihug. N Nitro Type | Compe.. E 3:28 Now playing. e QBA2 2020-2021 DO 12 13 14 15 16 17 18 19 20 21 21 of 21 Raymond went to the grocery store with his mom. He started by pushing an empty grocery cart with 0 kg of mass. As the shopping trip progressed, the cart increased in mass from 0 kg to 10 kg. Which argument below supports Raymond's claim that it was more difficult to push the 10 kg cart as opposed to the empty cart. O As the cart decreased in mass, Raymond had to use more force to push the cart. O As the cart increased in mass, Raymond had to use more force to push the cart. O As Raymond continued to push the cart during the duration of the trip, he became tired which made it more difficult to push the cart. O As Raymond continued to push the cart during the duration of the trip, friction began to build up on the wheels causing the cart to be more difficult to push.arrow_forward
- A 10.0-mL sample of alcohol is pipetted into a flask with stopper. The mass is found by difference to be 7.899 g. Refer to Example Excercise 1 and calculate the density of the liquid.arrow_forward# Page nanging units: Conversion Factor 3 E D 20 F3 $ If a car travels a distance of 241 km, what is that distance in miles? 4 888 R F % 5 T F5 G 6 MacBook Air F6 & Y H & 7 ◄◄ F7 U J * 00 8 | DII F8 35 K > of 63 ( 9 DD F9 O L ) O F10 P ;arrow_forwardPerform the following operation and express the answer in scientific notation. 2.60×10-5 × 2.10x10-5 [?]×10 21arrow_forward
- (a) To identify a liquid substance, a student determined itsdensity. Using a graduated cylinder, she measured out a 45-mLsample of the substance. She then measured the mass of thesample, finding that it weighed 38.5 g. She knew that the substancehad to be either isopropyl alcohol (density 0.785 g/mL)or toluene (density 0.866 g/mL). What are the calculated densityand the probable identity of the substance? (b) An experimentrequires 45.0 g of ethylene glycol, a liquid whose densityis 1.114 g/mL. Rather than weigh the sample on a balance, achemist chooses to dispense the liquid using a graduated cylinder.What volume of the liquid should he use? (c) Is a graduatedcylinder such as that shown in Figure 1.21 likely to afford theaccuracy of measurement needed? (d) A cubic piece of metalmeasures 5.00 cm on each edge. If the metal is nickel, whosedensity is 8.90 g/cm3, what is the mass of the cube?arrow_forward3. Perform the following calculations reporting your answer to the correct number of significant figures and with the correct units. a) 25.4 mL 21.4 mL = 1 1 kg b) 123.4 g * 1000 g c) 120.5 g + 32.567 g = conversion factors & calculations | EXP 2arrow_forwardDone < AA www-awu.aleks.com MEASUREMENT Multiplication and division of... Decide whether each proposed multiplication or division of measurements is possible. If it is possible, v last column of the table. proposed multiplication or division Is this possible? result x10 (2.0 mg) - (0.020 g) = ? yes ola no Ar yes (4.0 g) · (6.0 mL) = ? no yes (2.4 em) · (0.30 m) = ? %3D no Explanation Check © 2021 McGraw-Hill Education. All Rights Reserved.arrow_forward
- 2. Perform the indicated operations and give answers in the indicated unit and with the proper number of significant figures. (a) 113.137g-2.047 g 50.0 mL-37.40 mL (in g/mL) (b) 25.50 g +462 mg + 0.0100 kg (in g) (c) 0.900 mm * 0.8750 cm * 0.0031489 m (in cm³)arrow_forward[References] Use the References to access important values if needed for this question. A soda bottle is found to have a volume of 772 mL. Using unit analysis, show what the volume of this soda bottle is in L. Use one of the following to set up the conversion factor. 1 kg = 1000 g =1L Visited 100 cm=1 m 1000 mg=1g 3 1 mL=1 cm³ 1000 mm=1m 772 mL X (number) (unit) (number)(unit) Submit Answer L Try Another Version 1 item attempt remaining Previous Next>arrow_forwardAn electric current of 0.650 A flows for 1.0 second. Calculate the amount of electric, charge transported.. Be sure your answer has the correct unit symbol and 2 significant digits. 0 x10 ロ・ロ X μ = 5.:.arrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 Introductory Chemistry: A FoundationChemistryISBN:9781337399425Author:Steven S. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
Introductory Chemistry: A FoundationChemistryISBN:9781337399425Author:Steven S. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning


Introductory Chemistry: A Foundation
Chemistry
ISBN:9781337399425
Author:Steven S. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning
