
Chemistry
10th Edition
ISBN: 9781305957404
Author: Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Question
Transcribed Image Text:McGraw-Hill Education Campus x
A ALEKS- Natalie Drury - Learn
- eSantaFe | Santa Fe College | Ga X Sp This is the link to your ALEKS Ho X
A www-awn.aleks.com/alekscgi/x/lsl.exe/1o_u-lgNslkr7j8P3jH-IJgt8PIGunmDn7WsVrRAXK6XnHkiRvH2tl8o1N01pureF2mxffikZh47mlFqej2wRxcep33z_4FgzRgi30JbNyNsZVCgva
O CHEMICAL REACTIONS
Natali
Solving for a reactant in solution
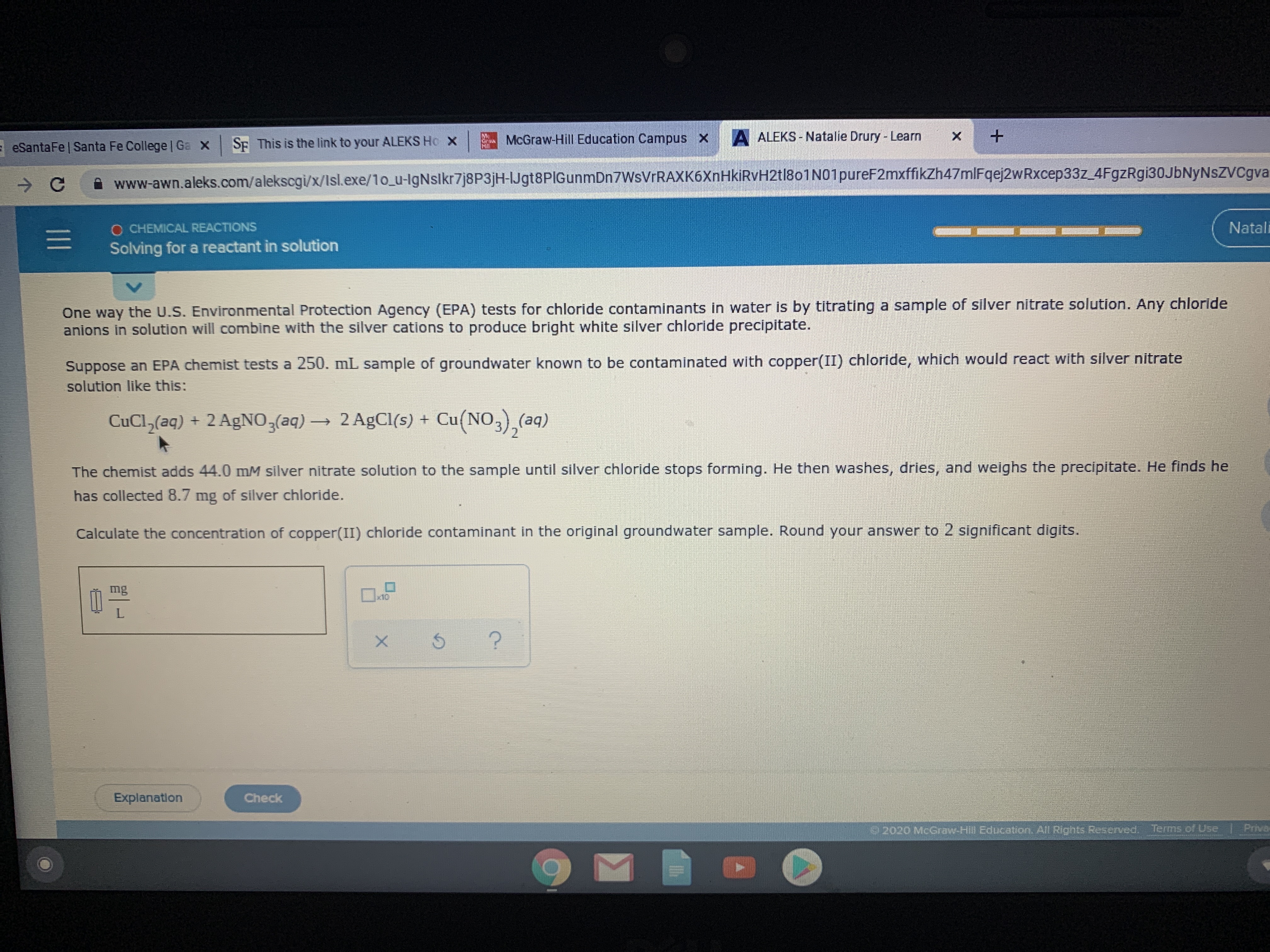
One way the U.S. Environmental Protection Agency (EPA) tests for chloride contaminants in water Is by titrating a sample of silver nitrate solution. Any chloride
anions in solution will combine with the silver cations to produce bright white silver chlorlde precipitate.
Suppose an EPA chemist tests a 250. mL sample of groundwater known to be contaminated with copper(II) chloride, which would react with silver nitrate
solution like this:
CuCl,(aq) + 2 AgNO,(aq)→ 2 AgCl(s) +
Cu(NO3),(aq)
The chemist adds 44.0 mM silver nitrate solution to the sample until silver chloride stops forming. He then washes, dries, and weighs the precipitate. He finds he
has collected 8.7 mg of silver chloride.
Calculate the concentration of copper(II) chloride contaminant in the original groundwater sample. Round your answer to 2 significant digits.
mg
x10
Explanation
Check
2020 McGraw-Hill Education. All Rights Reserved. Terms of Use
Priva
ना
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution
Trending nowThis is a popular solution!
Step by stepSolved in 2 steps

Knowledge Booster
Similar questions
- Commun x Bb Blackboard Collaborate Ultra -2 X General Psychology-Fall 20 O X A ALEKS - Griffin Barden - Learn ww-awa.aleks.com/alekscgi/x/Isl.exe/1o_u-IgNslkr7j8P3jH-JcZzdcvSCzsqTCIDqNGV3bKqhMfPmUcQ4ENkmiXn9QCwgeDPDkQ06yszYWESPcekwL0-Qg619rekU7404HgFAGBEZaDr080?1oBw7QYjibavbSPXtx-YCjsh_7mMmrq#item O THERMOCHEMISTRY Griffin V Using Hess's Law to calculate net reaction enthalpy Nitric acid can be formed in two steps from the atmospheric gases nitrogen and oxygen, plus hydrogen prepared by reforming natural gas. In the first step, nitrogen and hydrogen react to form ammonia: N,(g) + 3 H,(g) → 2 NH3(g) AH=-92. kJ In the second step, ammonia and oxygen react to form nitric acid and water: NH3(g) + 20,(g) HNO3(g) H,O(g) AH=-330. kJ – + Calculate the net change in enthalpy for the formation of one mole of nitric acid from nitrogen, hydrogen and oxygen from these reactions. dla Round your answer to the nearest kJ. kJarrow_forwardM Omail Youtube Maps tab = esc caps lock www-awu.aleks.com/alekscgi/x/1sl.exe/1o_u-IgNsikr7j8P3JH-IVTqeviKFP6W0cqJcWJdIACROQwyw24GWHinCUucXTT6KAMWthKMTgm0fQ2Bp9bAXgnoENSUD OSTATES OF MATTER Using heat of fusion or vaporization to find the heat needed to... L Calculate the amount of heat needed to boil 174. g of methanol (CH3OH), beginning from a temperature of 48.5 °C. Round your answer to 3 significant digits. Also, be sure your answer contains a unit symbol. 0 Explanation 1 1 Q A option Z @ 2 Check W S # 3 X H command E D X $ 4 C > R F Ś % 5 V I T G tv ^ 6 MacBook Pro B Thank Y H & 7 Ⓒ2022 McGraw Hill LLC. All Rights Reserved. Terms of Use | Privacy Center | Accessibility AK O ZAO Aa C U N J 8 The | M T 9 K ( a O < H 1 0 L command DIS O P option 11 T 2 2arrow_forwardhttps://umbc.realizeithome.com/X + ★Bookmarks ✰ umbc.realizeithome.com/RealizeitApp/Student.aspx?Token=oH0rNtSyBpgjW5t|6gBWmvpajONzIc8ql0d0PSGFSJFzsDZXGue0Aowa8qrSmF6MLCSor5bG%2bcVSyGjz1ynl33c58RoQvh8... Q 18 it JUMBC UMBC CHEM 102 / Unit 15: Spaced Practice Assessment 1/ Do assessment Unit 15: Spaced Practice Assessment 1 2 Question number 13 Predict whether the ionic compound Cr/3 will form an acidic, basic, or neutral solution when dissolved in water. You can assume the compound is completely soluble in water. basic acidic neutral 8 Next H Exit * = 0 Other bookmarks Egbe 7:03 PM 11/10/2022arrow_forward
- с www-awu.aleks.com/alekscgi/x/Isl.exe/10_u-IgNslkr7j8P3JH-IQUHIQg6bJxmeSyVPHOEB1plef9xy C5Ca9QIUX9FDNs1kHQvOMzcrVpгA IBP5q2HYwPcASZQKNla... Naming and Drawing Organic Molecules Recognizing different skeletal structures How many different molecules are drawn below? mxxx sex Explanation Check G MacBook Air 0/5 Julianna ? olo Ar 2024 McGraw Hill LLC. All Rights Reserved. Terms of Use | Privacy Center | Accessibilityarrow_forwardd ← Chrome esc e 24% (92) ALE X Mathwa X G 102 cels X ☆ www-awu.aleks.com/alekscgi/x/isl.exe/1o_u-IgNsikr7j8P3jH-IvTqeviKFP6W0cqJcWJdIACROQwyw24GWHinMCnqLGstAPM iZtogOAPGJIWPnLIY... 17.4 Solubility and... 18.3 Gibbs Free E... 5.3 Enthalpies of... 18.5 Gibbs Free E... Reading Schedule 19.6 Reduction Po... Y SOLUTION: The le... Math 115 W-S Fall.... File email d x Edit View History Bookmarks Profiles View 7. Q A Nancy X Z Explanation O KINETICS AND EQUILIBRIUM Using the Arrhenius equation to calculate k at one temperature... 2 W S The rate constant of a certain reaction is known to obey the Arrhenius equation, and to have an activation energy E-29.0 kJ/mol. If the rate constant of this reaction is 2.8 x 10³ Ms at 320.0 °C, what will the rate constant be at 222.0 °C? -1 Round your answer to 2 significant digits. Check X A ALEKS X CIChego X H command # 3 E Tab Window Help D $ 4 C X R > F 5 (92) ALE X % 5 V I T G #tv Welcom X A ALEKS ^ 6 MacBook Pro B Y We 9 "/ & 7 H X U N You 8 J 0000015 1…arrow_forward92% D Sun 3:04 PM OneLogin B McGraw-Hill Can O Question 1- 8.4 M MHE Reader B McGraw-Hill Cam x A ALEKS - Esther C x -> A www-awa.aleks.com/alekscgi/x/lsl.exe/1o_u-IgNslkr7j8P3jH-IBcn9hvCfbYq_fi3Zsn8H2oW_5PTMiOacVC-TPIQJ5aqZ8Tg8iSQs6Rzj49leNgLwH_Jfre.. ☆ -pps M Gmail O YouTube O Maps BC Broward College |.. O Sample Source An.. O New Tab G What does Duckw. E Untitled documen. B Reading List O KINETICS AND EQUILIBRIUM Using reactant reaction order to predict changes in initial rate Esther A certain reaction is first order in H, and first order in I,. Use this information to complete the table below. Be sure each of your answer entries has the correct number of significant digits. [4] [1] initial rate of reaction 1.25 M 1.39 M 15.0 M/s do 0.431 M 1.39 M 2.06 M 0.844 M Check Explanation O 2021 McGraw-Hill Education. All Rights Reserved. Terms of Use I Privacy | Accessibility 642 4 MacBook Airarrow_forward
- Edit View History Bookmarks Profiles Tab Window Help ALEKS = ALEKS - Tiffany Te-L-Peng - L X + www-awu.aleks.com/alekscgi/x/lsl.exe/1o_u-IgNslkr7j8P3jH-liGBdp5ulp5VqUnMDyPOVaX6q0CRPLZSQ5NeDIDYpEBUXdvV8IDfHNhBcWQZIM O ORGANIC OXIDATION AND REDUCTION Predicting the products of alkene ozonolysis X Predict the major products of this organic reaction. B Explanation Check c c C + T B 1.03 (CH3) S X MacBook Pro S Ć A Click and drag to start drawing a structure. O $ © 2022 McGraw Hill LLC. All Rights Reserved. Terms of Use | Privacarrow_forwardM Gmail T ALEKS - Rafia Riaz - Learn Significant Figures Calculator - Six b Home | bartleby www-awu.aleks.com/alekscgi/x/Isl.exe/1o_u-IgNslkr7j8P3jH-lvdw7xgLCkqMfg8yaFKbD9GafJstkYLIJnusCO2wDIf49ytsp8EECIGBi6veibDgHNs16W4StInReRBanAJb03txg-0axcq?1oBw7QYjlbavbS... ☆ YouTube Maps X C Calculate the amount of heat nee X Type here to search Translate Explanation News O STATES OF MATTER Using heat of fusion or vaporization to find the heat needed to... College information Check Calculate the amount of heat needed to melt 141. g of ice (H₂O) and bring it to a temperature of 54.1 °C. Be sure your answer has a unit symbol and the correct number of significant digits. 0 발 x10 X G Calculate the amount of heat nee X 9 1/3 + © 2023 McGraw Hill LLC. All Rights Reserved. Terms of Use | Privacy Center Earnings upcoming (32) Rafia V olo Ar Accessibility J ★ x Other bookmarks 10:52 PM 2/8/2023arrow_forwardCourse Home © planned parenthood - Google Se x + * openvellum.ecollege.com/course.html?courseld=17221551&OpenVellumHMACc191920409449ebe43741778f1605e61#10001 Students Welcome, Princesse P MLA Works Cited P G kenyon college - G. G university of dayton. G Xavier University of G howard university - G kent state - Google. O Schedule Appointm. R Red Straight Hair 1. Oral Health And M. 25 Crafts to Make a v Correct Part B If the volume of the original sample in Part A (P = 412 torr , Vị = 10.0 L ) changes to 64.0 L , without a change in the temperature or moles of gas molecules, what is the new pressure, P2? Express your answer with the appropriate units. > View Available Hint(s) HA P = Value Units %3D Submit de Feedback Next > 344 PM O 3/28/2022 39F Cloudyarrow_forward
- n Home Work-2 (page 7 of 8) b My Questions | bartleby A moodle.nct.edu.om/mod/quiz/attempt.php?attempt=709737&cmid=76257&page=6 E Apps * Bookmarks تحويل كيلومتر إلى م. .. © E Reading list NCT e-Learning Portal Courses Reports - e-Services Academic Departments - ETC - CIMS - Muayid Mahmood Mohammed Al Azri Fundamentals Of Chemistry (Engineering) Dashboard / My courses / CHEM1100 / Home works / Home Work-2 Quiz navigation Question 7 How many Faraday is needed to deposit 1.8 g of Sodium (Na) from NaCl solution using electrolysis process. Not yet 1 2 3 4 5 6 8 answered Answer: Marked out of Finish attempt . 1.00 P Flag question Time left 191:45:56 Previous page Next page - Submission Link for Home Work-1 Jump to. Quiz 1 for Section-3 - You are logged in as Muayid Mahmood Mohammed Al Azri (Log out) CHEM1100 Data retention summary. Get the mobile app 10:13 PM P Type here to search 72°F Clear O E 1 G 4) ENG 12/14/2021arrow_forwardHelp 100% 47 T. "ublic Health Ch HSC 258 - Major Projec x MindTap - Cengage Lea X C The Illustration To T =55750828934189288909969212&elSBN=9781305657571&id=D1061392007&nbld=21... * Q Search t Referonces Use the References to access important values if needed for this question. For the following reaction, 50.4 grams of sulfur dioxide are allowed to react with 17.9 grams of water. sulfur dioxide (g) + water (I) sulfurous acid (H2SO3) (g) grams What is the maximum amount of sulfurous acid (H,SO3) that can be formed? What is the FORMULA for the limiting reagent? grams What amount of the excess reagent remains after the reaction is complete? Submit Answerarrow_forward°F rtly cloudy m/alekscgi/x/Isl.exe/1o_u-lgNslkr7j8P3jH-lvWyv8WYLP6W0cqJcWJdIACROQwyw24GWHInMM72ts 1 NTBcSzwZOGGhrmIP4yCejeRhX9HIzqlzRTj2iaDBXTCRIHYWNO_-o?10... O CHEMICAL REACTIONS Calculating molarity using solute moles A chemist prepares a solution of mercury(II) iodide (HgI₂) by measuring out 0.0161 μmol of mercury(II) iodide into a 300. mL volumetric flask and filling the flask to the mark with water. Calculate the concentration in μmol/L of the chemist's mercury(II) iodide solution. Round your answer to 3 significant digits. µ mol L Explanation Check x10 X Ś Q Search 2023 McGraw Hill LLC. All Rights Reserved. Terms of Use **************** D 1/5 www Jessi Privacy Center | Accessibilityarrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY