
Chemistry
10th Edition
ISBN: 9781305957404
Author: Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Concept explainers
Question
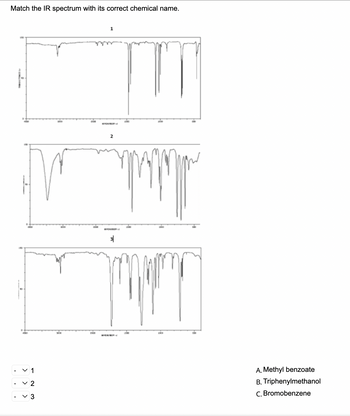
Transcribed Image Text:Match the IR spectrum with its correct chemical name.
1
2
ག། །
-
་་་་་
✓ 1
✓ 2
v3
A. Methyl benzoate
B. Triphenylmethanol
C. Bromobenzene
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution
Trending nowThis is a popular solution!
Step by stepSolved in 3 steps with 1 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- Ff.163.arrow_forwardCounterfeit drugs are a common problem in developing regions of the world. Oftentimes, counterfeit pills are made with compounds such as lactose. A lab technician has obtained the IR spectrum shown above for a sample reported to be citalopram, an antidepressant drug. Does the IR spectrum belong to citalopram or lactose? Explain your answer by describing what feature of the IR spectrum confirms your choice and describe what feature is missing from the IR spectrum for the other compound. A. citalopram B. lactosearrow_forwardDetermine the structures of these spectrums. (C4H10O has an unsaturation of 1) (C8H14O4 has an unsaturation of 4)arrow_forward
- Which of the following statements is correct? O A. Atomic emission detector is suitable for analyzing hydrocarbon compounds. B. The best carrier gas for a thermal conductivity detector is nitrogen. O C. Chlorine containing compounds are best analyzed by using an electron capture detector. O D. Equimolar amount of C6H14 and C3H8 should produce the same signals using a flame ionization detector. O E. Mass spectrometry detector is nondestructive.arrow_forwardWhich atom do you expect is present in this spectrum? 100 Rel. Intensity 80 60 60 40 40 20 20 0.0 0.0 15 30 45 60 75 90 m/z NIST Chemistry WebBook (https://webbook.nist.gov/chemistry) Only hydrocarbons Bromine Chlorine O Nitrogenarrow_forward4. Ethyl acetate and 2-butene-1,4-diol both have the molecular formula C4H8O2. How would you use infrared spectroscopy to distinguish between the two? Provide a thorough explanation.arrow_forward
- 10. Predict the number of signals expected (disregarding splitting) in the 1H spectrum of а. OH b. .CI HOarrow_forward4. For each example below, draw at least onc possīble isomer that is consistent with the molecular formula and the associated IR spectrum. Page 3 a. MW 82, CaH1O be 48 20 1000 b. MW 116, Coll2O2 4000 2540 SL LOB2 9262 2042arrow_forward
arrow_back_ios
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY