
Chemistry
10th Edition
ISBN: 9781305957404
Author: Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Concept explainers
Question
thumb_up100%
Please see image
Transcribed Image Text:ome
Uws About the Program |
Mi
A www-awu.aleks.com/alekscgi/x/lsl.exe/1o_u-lgNslkr7j8P3jH
MEASUREMENT AND MATTER
1/3
Setting up the math for a one-step problem with uni...
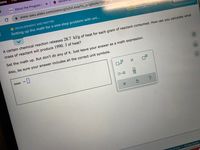
A certain chemical reaction releases 28.7 kJ/g of heat for each gram of reactant consumed. How can you calculate what
mass of reactant will produce 1990. J of heat?
Set the math up. But don't do any of it. Just leave your answer as a math expression.
Also, be sure your answer includes all the correct unit symbols.
mass
Ar
ms of Use Privacy Center
olo
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by stepSolved in 2 steps with 2 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- Edit View History Bookmarks Profiles Tab Window Help ome File d Dashboard ALEKS - Reyna Garcia - Lear Watch Gilmore Girls | Netflix A www-awu.aleks.com/alekscgi/x/lsl.exe/1o_u-IgNslkr7j8P3jH-IVDWKW_BBZZ16tTytly4Fcfu6zOtC Spotify Web Playe... M Common Ethical D... O CHEMICAL REACTIONS Writing a chemical equation from a description of the reaction Solid potassium and chlorine gas combine to produce solid potassium chloride. Write a balanced chemical equation for this reaction. Explanation Check FEB 2arrow_forwardMALEKS M Gmail ▬▬ 个 YouTube a X ALEKS - Rafia Riaz - Learn X Maps D Translate www-awu.aleks.com/alekscgi/x/Isl.exe/1o_u-IgNslkr7j8P3jH-lvdw7xgLCkqMfg8yaFKbD9GafJstkYLIJnusWtY-MBckoyqj9qFtHEkTFh5e-b9D0Ng1h9QM42... Q O ACIDS AND BASES Predicting acid or base strength from the conjugate News species H₂O Explanation Bro Order these chemical species by increasing pH of an 0.1 M aqueous solution of each. That is, imagine making an 0.1 M solution of each species. Select 1 next to the species that makes the solution with the lowest pH. Select 2 next to the species that makes the solution with the next higher pH, and so on. H₂0* HF Notice that some of the rankings have been filled in for you already. Also notice that water is on the list. For that particular case, just compare the pH of pure water to the pH of the other solutions. Note for advanced students: for all charged species, you may assume the necessary counterions act as neither acids nor bases. CH₂CH₂COOH b Answered: O ACIDS AND HBrO…arrow_forwardme File Edit View History Bookmarks Profiles Tab Window Help Watch Gilmorex * Dementia Frien x 1 unread) - dte X Lobby Top Hat X 2 Dashboard xV Lives Well Livec x A ALEKS - David x Psychology Re i www-awu.aleks.com/alekscgi/x/lsl.exe/1o_u-IgNslkr7j8P3jH-IVDWKW_BBZZ6tTytly4Fcfu6zOtOf8oMM9s8f0Bcp47VrzRTjh-0_CAFLD-fp-V... Spotify Web Playe.. M Common Ethical D.. O CHEMICAL REACTIONS Using a chemical equation to find moles of product from moles ... Dav Gaseous ammonia chemically reacts with oxygen (0,) gas to produce nitrogen monoxide gas and water vapor. Calculate the moles of water produced by the reaction of 0.700 mol of oxygen. Be sure your answer has a unit symbol, if necessary, and round it to the correct number of significant digits. Explanation Check © 2022 McGraw Hill LLC. All Rights Reserved. Terms of Use | Privacy Centerarrow_forward
- W AutoSave On 2203 assignment - Compatibility Mode • Saved - O Search (Alt+Q) raghav grover RG File Home Insert Draw Design Layout References Mailings Review View Help P Comments A Share O Find Times New Roman v 10 - A A Aa - A Normal Body Text List Paragraph No Spacing E Replace Paste В I U v ab x, x A - Dictate Editor Reuse A Select v Files Undo Clipboard s Font Paragraph Styles Editing Voice Editor Reuse Files 1. Provide the missing reagents for each of the following reactions: CH2 но он ´HO CH,NH2 Ph CI Accessibility: Unavailable D Focus Page 1 of 19 259 words English (United States) 72% ENG 11:37 AM O Type here to search W 1°C Cloudy US 2022-04-20arrow_forwardW AutoSave On chem - Compatibility Mode • Saved - O Search (Alt+Q) raghav grover RG File Home Insert Draw Design Layout References Mailings Review View Help P Comments A Share O Find Times New Roman v 12 - A A Aa v A Normal Body Text List Paragraph No Spacing E Replace Paste В I U ab x, x A - Dictate Editor Reuse A Select v Files Undo Clipboard s Font Paragraph Styles Editing Voice Editor Reuse Files Q6. Provide a step-wise synthesis for the following compound using benzene as a starting material. Br `NH2 K Accessibility: Unavailable D'Focus Page 4 of 13 523 words English (United States) 16 ENG 6:17 PM O Type here to search -16°C US 2022-03-02 近arrow_forwardW AutoSave On assignment - Compatibility Mode • Saved - O Search (Alt+Q) raghav grover RG File Home Insert Draw Design Layout References Mailings Review View Help P Comments A Share O Find Times New Roman v 12 - A A Aa v A Normal Body Text List Paragraph No Spacing E Replace Paste В I U v ab x, x A - Dictate Editor Reuse A Select v Files Undo Clipboard a Font Paragraph Styles Editing Voice Editor Reuse Files Using the following starting materials as the only source of carbon, provide a synthesis of the product shown below. You may use any reagents that you require. 14. OMe Br MeO Accessibility: Unavailable DFocus Page 13 of 19 258 words English (United States) 100% ENG 9:11 AM O Type here to search 1°C Cloudy US 2022-04-20 21arrow_forward
- Chrome File Edit View History Bookmarks People Tab Window Help 78% O Thu 10:02 PM A ALEKS - Jacqueline Hoppenrey x A www-awn.aleks.com/alekscgi/x/Isl.exe/1o_u-IgNslkr7j8P3jH-IQİHQRDYV_6Ux63SypJXz0Coxvwqgg4JkWI7XM99oekXTcojkLT31OZqp4772m0XOsdozQ5qMD3aRdEyOGQe33sgOsaJ. O GASES Calculating partial pressure in a gas mixture Jacqueline A 5.00 L tank at 7.37 °C is filled with 18.2 g of chlorine pentafluoride gas and 3.28 g of boron trifluoride gas. You can assume both gases behave as ideal gases under these conditions. Calculate the mole fraction and partial pressure of each gas, and the total pressure in the tank. Round each of your answers to 3 significant digits. 圖 mole fraction: chlorine pentafluoride partial pressure: | atm mole fraction: boron trifluoride partial pressure: | atm Total pressure in tank: | atm Explanation Check O2021 McGraw-Hill Education All Rights Reserved Terms of Use Privacy Accessibility MacBook Air 山回arrow_forwardEdit View History Bookmarks Profiles Tab Window Help с ALEKS M X A ALEKS - Tiffany Te-L-Peng - Le X + www-awu.aleks.com/alekscgi/x/lsl.exe/1o_u-IgNslkr7j8P3jH-liGBdp5ulp5VqUnMDyPOVaX6q0CRPLZSQ5NDHtEP4AK_F O APPLICATIONS OF SUBSTITUTION AND ELIMINATION Predicting the products of nucleophilic epoxide ring opening Add the necessary reaction conditions above or below the arrow in this organic reaction. Also, if a major product is missing from the right-hand side, draw it in. C+ C™ C + Explanation Check B X T Ś HO Ć F Br Ⓒ2022 McGraw Hill LLC. All Rights Rearrow_forwardHelp 100% 47 T. "ublic Health Ch HSC 258 - Major Projec x MindTap - Cengage Lea X C The Illustration To T =55750828934189288909969212&elSBN=9781305657571&id=D1061392007&nbld=21... * Q Search t Referonces Use the References to access important values if needed for this question. For the following reaction, 50.4 grams of sulfur dioxide are allowed to react with 17.9 grams of water. sulfur dioxide (g) + water (I) sulfurous acid (H2SO3) (g) grams What is the maximum amount of sulfurous acid (H,SO3) that can be formed? What is the FORMULA for the limiting reagent? grams What amount of the excess reagent remains after the reaction is complete? Submit Answerarrow_forward
arrow_back_ios
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY