
Chemistry
10th Edition
ISBN: 9781305957404
Author: Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Question
Q14
Needed to be solved both part of the questions correctly in the order to get positive feedback please show me neat and clean work for it by hand solution needed

Transcribed Image Text:Macmillan Learning
a) Predict the major organic product of treating the 3-methylbutene with hydrogen bromide in the presence of peroxides.
HBr
ROOR
Describe the major organic product.
product
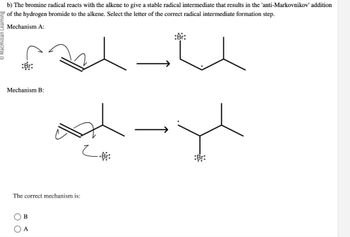
Transcribed Image Text:b) The bromine radical reacts with the alkene to give a stable radical intermediate that results in the 'anti-Markovnikov' addition
of the hydrogen bromide to the alkene. Select the letter of the correct radical intermediate formation step.
Mechanism A:
:Br:
Mechanism B:
The correct mechanism is:
(B
A
:Br:
og-yl
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution
Trending nowThis is a popular solution!
Step by stepSolved in 4 steps with 2 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- Hello, Can you please answer these 3 questions? Thank you!arrow_forwardSaved Normal BIIIU fxl Ix Actual mass used in solution prep (g) Volume solution prepared Measured conductivity (uS/cm) Substance 0.1 g Naci 0.1003 100 mL 1149 0.1 g Nal 0.10 100 ml 419 Calculate the mass of Nal that would be necessary to yield the same conductivity as the NaCl solution. Clearly show these calculations in your lab notebook. Saved T BII U X X + fr Normalarrow_forward12 of 19 <. I Review | Constants | Periodic Table Part B Before investigating the scene, the technician must dilute the luminol solution to a concentration of 4.00×10-2 M. The diluted solution is then placed in a spray bottle for application on the desired surfaces. How many moles of luminol are present in 2.00 L of the diluted spray? Express your answer with the appropriate units. » View Available Hint(s) moles of luminol = Value Units %3Darrow_forward
- Please show work, just like I need to put it in. Make clear HOW you got to that answer. Please write neat & make it readable. Again please write like I would need to put it in!arrow_forwardA stock solution whose concentration is 25.00 mg/mL was provided. A primary standard solution was then prepared by transferring 5.00 mL of the stock solution into a 50.00 mL volumetric flask. The primary standard solution was used to prepare three standard solutions of different concentrations by taking different volumes of aliquots into three 25.00 mL volumetric flasks: Standard Solution #1 #2 #3 Amount of Primary Solution (mL) 5.00 10.00 15.00 Three 25.00 mL volumetric flasks were then diluted to mark with water. Questions #1 What is the concentration of the primary standard solutions, expressed in units of mg/ml? #2 Re-write the concentration of the primary standard solutions obtained from Q#1 in units of μg/mL. #3 Determine the concentrations of three standard solutions in units of µg/mL. #4 What is a requirement for a "blank" solution used in calibration in spectrophotometry? Page 5 (Experiment #3)arrow_forwardA student prepares a 1.1 mM aqueous solution of 4-chlorobutanoic acid (C3H, CICO,H). Calculate the fraction of 4-chlorobutanoic acid that is in the dissociated form in his solution. Express your answer as a percentage. You will probably find some useful data in the ALEKS Data resource. Round your answer to 2 significant digits. % X Sarrow_forward
- A student prepares a 0.20M aqueous solution of acetic acid (CH3CO₂H). Calculate the fraction of acetic acid that is in the dissociated form in his solution. Express your answer as a percentage. You will probably find some useful data in the ALEKS Data resource. Round your answer to 2 significant digits. % 0 ☐x10 xarrow_forwardBalance each of the following neutralization reactions. Part A HNO, (aq) + Sr(OH)2(s)→H20(1) + Sr(NOs)2(aq) Express your answer as a chemical equation. Identify all of the phases In your answer. ΑΣφ ? DA chemical reaction does not occur for this question. Submit Request Answer t Speec.pdfarrow_forwardI need help calculating the initial concentrations please.arrow_forward
arrow_back_ios
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY