
Chemistry
10th Edition
ISBN: 9781305957404
Author: Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Question
A sample (50 mL) of a bottled water was added to a 100 mL volumetric flask and then diluted to the mark. From this stock solution, 50 mL was taken, added to a 100 mL volumetric flask and diluted to the mark. Final concentration of the serial dilution: 587.7161ppm.
1. what is the concentration in the original bottle (ppm)
Expert Solution
arrow_forward
Step 1
The dilution takes place twice. First the original sample (50 mL) is diluted to 100 mL stock solution. Then 50 mL of stock solution is diluted to 100 mL solution with concentration = 587.7191.
Concentration of the stock solution can be determined using the expression for dilution is,
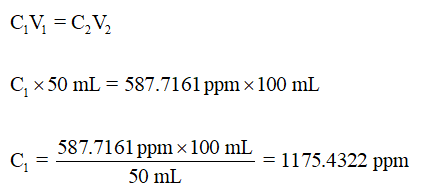
Trending nowThis is a popular solution!
Step by stepSolved in 2 steps with 2 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- Someone is attempting to measure the H3PO4(aq) content of an unknown solution by both titration with NaOH(aq) and calorimetry. The person measures 100 mL of stock NaOH(aq) solution into a buret but forgets to write down its concentration. They then measure out 50.0 mL of unknown H3PO4(aq) solution (which has an indicator) and places this solution in a constant-pressure calorimeter for the titration experiment. The initial temperature of all the solutions is 22.9 Celsius. The student adds 46.84 mL of NaOH(aq) from the buret to the H3PO4(aq) solution in the calorimeter to reach the equivalence point (color change for indicator). The final solution temperature is 33.5 Celsius. Assume density and specific heat of all solutions are the same as that of pure water and that volumes are additive. a) what is the balanced molecular equation for the acid-base reaction that takes place in the calorimeter? b) Calculate delta HRXN (enthralpy) for the above acid-base reaction c) what is the…arrow_forwardConsider a 0.227 M aqueous solution of sodium hydroxide, NaOH. A. How many grams of NaOH are dissolved in 22.82 mL? B. How many moles of hydroxide ions (OH-) are found in 22.82 mL? C. How many moles of sulfuric acid, H2SO4, are neutralized by 22.82 mL of 0.227 M NaOH(aq)?[Hint: begin by writing a balanced chemical equation for this neutralization reaction.]arrow_forward1. What is the concentration in ppm if 0.808 grams of CaCl2 is dissolved in 250 ml of water. A. 11220 ppmB. 3220 ppmC. 2330 ppmarrow_forward
- You are standardizing a solution of KOH. You weigh out 2.34 g of potassium hydrogen phthalate, dissolve it in water and add 2 drops of phenolphthalein solution. You fill the buret with the KOH solution to an initial reading of 2.33mL. You add the KOH solution to the flask and when the endpoint is reached you record the final buret reading to be 34.11mL. Calculate the molarity of KOH in the solution.arrow_forwardWhat is the minimum volume of 2.19 mol L−1 HCl(aq) required to dissolve 16.7 g Zn metal? The atomic weight of Zn is 65.39 g mol−1. Zn(s) + HCl(aq) ⟶⟶ ZnCl2(aq) + H2(g) (unbalanced) Enter your answer accurate to three significant figures.arrow_forwardYou are washing glassware in a lab. The solution your 1 L beaker contains 5 mol/L NaOH in water. It is known that 1% of the mixture adheres to the walls of the beaker when the beaker is drained. Thus, if you drain the beaker, 0.01 L of the original contents of the beaker remains in the beaker. The beaker must be filled with 1 L of pure water between each rinse. The beaker is then stirred very well. What is the composition of the contents of the beaker, in mol/L, if it is rinsed once? How many rinses does it take to get the contents of the beaker to below 0.00005 mol/L?arrow_forward
- A student prepares a dilute solution of sodium hydroxide, NaOH (aq), starting with 6 M sodium hydroxide. She then titrates a 1.372 g sample of KHP with the dilute sodium hydroxide solution, NaOH (aq), to a phenolphthalein end point. A.) If the titration required 21.84 mL of sodium hydroxide, NaOH (aq), calculate the molar concentration of the sodium hydroxide solution, NaOH (aq). (Remember that KHP is potassium hydrogen phthalate, KHC8H4O4, NOT potassium hydrogen phosphorus!) B.) The student uses the same sodium hydroxide to titrate 10.00 mL of vinegar to a phenolphthalein end point. If the titration required 27.48 mL of sodium hydroxide, NaOH (aq), calculate the molar concentration of acetic acid, HC2H3O2 (aq), in the vinegar. C.) Calculate the mass percent of acetic acid, HC2H3O2 (aq), in the vinegar using the molar concentration for acetic acid, HC2H3O2 (aq), determined in part b and assuming the density of the solution is 1.01 g/mL.arrow_forwardHow to prepare these solutions? 250.0 mL 0.125 M stock Na2S2O3 solution from Na2S2O3·5H2O crystals 250 mL 0.10 M NaOHNOTE: Use the 1.0 M NaOH prepared250.0 mL standard 2500 ppm Cu(II) stock solutiona. Weigh and dissolve appropriate amount of Cu(NO3)2·5H2O crystals in enough distilled water.arrow_forwardA student prepares a 0.29 M aqueous solution of propionic acid (C₂H,CO₂H). Calculate the fraction of propionic acid that is in the dissociated form in his solution. Express your answer as a percentage. You will probably find some useful data in the ALEKS Data resource. Round your answer to 2 significant digits. % Xarrow_forward
- POSTLABORATORY ASSIGNMENT 1. An oleic acid, C17H33COOH (282 g/mol), solution is added to water in a watchglass until a monolayer forms. Assume that there are no spaces between molecules in the monolayer and that each oleic acid molecule occupies an area of 0.25 nm². If the concentration of the oleic acid solution is 0.00012 g/mL, what is the experimental value of Avogadro's number? dropper pipet calibration number of drops of oleic acid in the monolayer diameter of monolayer OMA 30+20 od os pulgad Im od of ingranit yd souls mesto bonstantial = || || || 00.1 arestusslom (0) N = = = Jorna ode 65 drops/mL 16 drops 14.5 cm dirua orli unalools) mano lo zemoristalls d A(otho) wallarrow_forwardA chemistry student is given 1.00 L of a clear aqueous solution at 17 degrees Celsius. He is told an unknown amount of a certain compound X is dissolved in the solution. The student allows the solution to cool to 17 degrees Celsius. The solution remains clear. He then evaporates all of the water under vacuum. A precipitate remains. The student washes, dries and weighs the precipitate. It weighs 0.12 kg. Using only the information above, can you calculate the solubility of X in water at 17° C? If you said yes, calculate it and be sure your answer has a unit symbol and 2 significant digits.arrow_forward< 11 of 19 I Review Constants | Periodic Table Part B A student placed 16.0 g of glucose (C6H12O6) in a volumetric flask, added enough water to dissolve the glucose by swirling, then carefully added additional water until the 100. mL mark on the neck of the flask was reached. The flask was then shaken until the solution was uniform. A 45.0 mL sample of this glucose solution was diluted to 0.500 L. How many grams of glucose are in 100. mL of the final solution? Express your answer to three significant figures and include the appropriate units. • View Available Hint(s) ? Value Unitsarrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY