
Chemistry
10th Edition
ISBN: 9781305957404
Author: Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Concept explainers
Question
What is the concentration of FeSCN2+ in each standard solution? (Show ALL calculations for each
solution) Stock Solution of the concentration of FeSCN^2+ is 0.00200 M
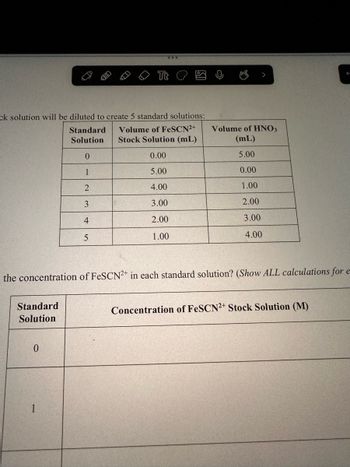
Transcribed Image Text:ck solution will be diluted to create 5 standard solutions:
Standard Volume of FeSCN2+
Solution Stock Solution (mL)
0
0.00
1
5.00
2
4.00
3
3.00
4
2.00
5
1.00
EN
Standard
Solution
the concentration of FeSCN2+ in each standard solution? (Show ALL calculations for e-
0
Volume of HNO3
(mL)
5.00
0.00
1.00
2.00
3.00
4.00
Concentration of FeSCN2+ Stock Solution (M)
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution
Trending nowThis is a popular solution!
Step by stepSolved in 5 steps with 7 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- Some wastewater stream contains 0.015 M Hg22+. Some chemical engineer decides to remove it by precipitating it as Hg2CO3(s). He mixes 100.00 ml of the wasterwater with 100.00 ml of a 2.00 M solution of Na2CO3 and filters the result to remove the solid. How much residual Hg22+ should he expect to find still in the water? (Hint: it should not be 0) Ksp of. Hg2CO3 = 9.0 x 10-15. Also keep in mind that Hg+ is the only ion that exists in solutions as a dimer, i.e. Hg22+, not 2 Hg+ (Hg2CO3→ Hg22+ + CO32-)arrow_forwardA solution contains 0.0450 M Ca2+ and 0.0940 M Ag+. If solid Na3PO4 is added to this mixture, which of the phosphate species would precipitate out of solution first? Ca3(PO4)2 Ag3PO4 Na3PO4 When the second cation just starts to precipitate, what percentage of the first cation remains in solution? percentage: 13.93 Incorrect %arrow_forwardA quantitative analysis for ethanol, C2H60, is accomplished by a redox back titration. Ethanol is oxidized to acetic acid, C2H402, using excess dichromate, 3+ Cr207 2-, which is reduced to Cr3*. The excess dichromate is titrated with Fe2*, giving C and Fe+ as products. In a typical analysis, a 5.00-mL sample of a brandy is diluted to 500 mL in a volumetric flask. A 10.00-mL sample is taken and the ethanol is removed by distillation and collected in 50.00 mL of an 2- acidified solution of 0.0200 M K2Cr207. Titration of the unreacted Cr207 requires 21.48 mL of 0.1014 M Fe2*. Calculate the %w/v ethanol in the brandy. Which is the titrant in this problem? C2H402 Fe2+ C2H60 O Cr207 2-arrow_forward
- 25.00 mL of a solution containing KIO4 is treated with excess potassium iodide. IO4- + 8H+ + 7I- → 4I2 + 4H2O The resulting solution is titrated with 0.15 M sodium thiosulfate pentahydrate. The average titre was 21.76 mL. I2 + 2S2O32- → 2I- + S4O62- What mass of KIO4 (in g) was dissolved in water to make the original solution?arrow_forwardDetermine the molarity of the 10.00 mL standard EDTA solution titrated using 1.000 g zinc sulfate in 100 mL solution. (ZnSO4 = 161.47 g/mol ) 0.006193 M 0.6193 M 6.193 M 0.06193 Marrow_forward(buffer )Find a buffer to maintain a laboratory system for coagulation at a pH of 5.5. The system uses a ferric chloride coagulant and generates acidity as defined by the reaction in equation (1). If the coagulant dose is 10 mg/L Fe+3 calculate the concentration of the buffer to maintain the pH at a level within 0.2 pH units. FeCl3 + 3 H2O ↔ Fe(OH)3 (s) + 3H+ + 3Cl-arrow_forward
- When ammonium sulfate dissolves, both the cation and the anion have acid-base reactions: (NH4)2SO4(s) = 2NH4* + SO,2 Ksp = 276 NH4 + H20 = NH3(aq) + H3O* Ka = 5.70 x 10 10 %3D SO,2- + H20 = HSO4 + OH Kp = 9.80 x 10-13 %3Darrow_forwardCalculate the concentration (in molarity) of free Cd2+ ions in a solution made by adding 0.35 moles of Cd(NO3)2 to 1.0 L of 6.0 M NaBr. Kf of (CdBr42−) = 5.0 xarrow_forward17.69 mL of the I2 solution from above is required to titrate a sample containing As2O3. Calculate the mass of As2O3 (197.8 g/mol) in the sample. As2O3 + 5(H2O) + 2I2 → 2(H3AsO4) + 4HIarrow_forward
- The balanced equation for the titration of oxalic acid with potassium permanganate is: 2 KMnO4 + 5 H2C2O4 + 3 H2SO4 → 2 MnOS4 + 10 CO2 + K2SO4 + 8 H2O What is the concentration of a 25 mL oxalic acid solution that requires 12.4 mL of a 0.0384 M KMnO4 solution to reach the endpoint of the titration? (report your answer to 4 decimal places)arrow_forwardYou are performing an experiment in lab that involves the titration of 25.0 mL of H2SO4 solution. You titrate the acidic solution with 0.8067 M NaOH and the equivalence point is reached by the addition of 17.31 mL of NaOH solution. Using the balanced equation below, calculate the molartity of H2SO4 in the flask. Do NOT include units. 2NaOH(aq) + H2SO4(aq) --> 2H2O(l) + Na2SO4(aq)arrow_forwardWhen a strong base is added to a solution of CuSO4, which is pale blue, a precipitate forms and the solution above the precipitate is colorless Part 1 See Periodic Table Include phases in the balanced chemical equations. What is the net chemical equation that describes this reaction? Cu+2(aq)+2OH−(aq)Cu(OH)2(s) Part 2 When ammonia is then added, the precipitate dissolves and the solution turns a deep navy blue. What is the net chemical equation that describes this event?arrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY