
Chemistry
10th Edition
ISBN: 9781305957404
Author: Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Question
Look at the structure of a soap molecule, and explain what happened when you added soap to your oily hands and put your hands under the water for the solubility lab. Why could you wash the oil off your hands with soap and water? You should use chemistry concepts including structure, polarity, and intermolecular forces to explain your answer.
attached is the lewis structure of a basic soap molecule. There are covalent bonds between all carbons, hydrogens, and oxygens. However, there is an ionic bond between oxygen and sodium ions.
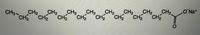
Transcribed Image Text:CH2
CH2
CO Na+
CH2
CH3-CH2
CH2
CH2
CH2
CH2 CH2
CH2
CH2
CH
CH2
CH2
CH
CH
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution
Trending nowThis is a popular solution!
Step by stepSolved in 2 steps with 3 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- Water has many exceptional and useful properties. Which is the rarest property among compounds? Water has a high heat capacity. Solid water is less dense than liquid water. Water is a solvent. Water has surface tension.arrow_forwardWhy can dissolved ionic compounds conduct an electric current but dissolved covalent compounds cannot?arrow_forwardWhat is the greenhouse effect? How does the excess CO2 gas in the atmosphere cause the greenhouse effect?arrow_forward
- Does NaCl(s) conduct electricity at room temperature? Yes/No and Explain why?arrow_forwardUsing the patterns evident in a table of electronegativity values, K has a higher electronegativity relative to Ca. True or False True Falsearrow_forwardDraw a water molecule and show the partial charges. Explain why water has a partial charge.arrow_forward
- we would predict that the imaginary ionic compound XF would have a (higher /lower) lattice energy than YyF2 becausearrow_forwardConsider the element Work (Wr) with an atomic number of 33 and an atomic mass of 81.03 g/mol. The following information was collected for this element Molecule/ Ion Names Wr-2 Workide WrO3-2 Workate WrO2-2 Workite WrO-3 hydroworkite Draw the Lewis structure of the molecule Workous acid and determine its electron geometry and molecular geometry. What is the mass % of work in Calcium hydroworkite.arrow_forwardUnderstanding electrostatic forces. Choose where the object outlined in green will go based on the charges.arrow_forward
- Use Lewis dot symbols to show the formation of magnesium oxide.arrow_forwardPredicting whether molecules are polar or nonpolar.arrow_forward2. Sulphur reacts with oxygen to form sulphur trioxide through sulphur monoxide and sulphur dioxide. Sulphur trioxide reacts with water to form sulphuric acid. Balance the equations for the above and draw the Lewis structures for each of the oxides and the acid. next pagearrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY