
Chemistry
10th Edition
ISBN: 9781305957404
Author: Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Concept explainers
Question
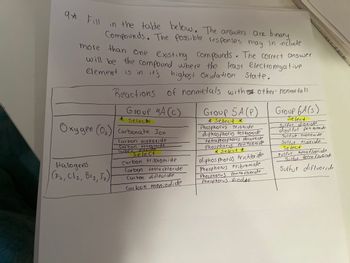
Transcribed Image Text:9* Fill
in the table below. The answers are binary,
Compounds. The Possible responses may in include
more than one existing compounds. The correct answer
will be the compound where the least Electronegative
element is in it's highest Oxidation State.
Reactions of nonmetals with other' nonmetals
Group HA (C)
Halogens
(F₂, Cl₂, B1₂, 5₂)
* Selec*
Oxygen (0₂) Carbonate Jon
Carbon monoxide
Carbon tetraoxide
Carbon
dioxide
Select
Carbon tribromide
Carbon tetrachloride
Carbon diflouride
Carbon moniodide
Group 5A (P)
* Select *
Phosphorus trioxide
diphosphorus tetraoxide
tetraphosphorus decaoxide
Phosphorus Pentaoxide
* Select *
diphosphorus trichloride
Phosphorus tribromide
Phosphorus Penta fluoride
Phosphorus diiodide
Group 6A(s)
Select
Sulfur dioxide
disulful penta oxide
Sulfut monoxide
Sulfur trioxide
Select
sulfur hexafluoride
Sulfur tetra fluoride
Sulfur difluoride
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by stepSolved in 4 steps

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- When each of the following pairs of aqueous solutions is mixed, does a precipitation reaction occur? If so, write the formula and name of the precipitate. (Type your answer using the format CO2 for CO2. Type NONE in the blanks if there is no precipitation reaction.) (a) lead(II) nitrate + potassium bromideformulaname(b) sodium sulfide + nickel(II) sulfateformulanamearrow_forwardsodium fluoride and aluminum sulfate Write a balanced molecular equation, including all physical states. Write a balanced total ionic equation, including all physical states and charges for individual ions.Write a balanced net ionic equation, including all physical states and charges for individual ions.arrow_forwardWhy are most covalent compounds non electrolytes? Explain using your own words, 2-4 complete sentences, and proper spelling and grammar.arrow_forward
arrow_back_ios
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY