
Chemistry
10th Edition
ISBN: 9781305957404
Author: Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Question
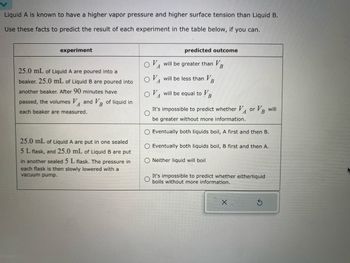
Transcribed Image Text:Liquid A is known to have a higher vapor pressure and higher surface tension than Liquid B.
Use these facts to predict the result of each experiment in the table below, if you can.
experiment
25.0 mL of Liquid A are poured into a
beaker. 25.0 mL of Liquid B are poured into
another beaker. After 90 minutes have
passed, the volumes V and VB of liquid in
A
each beaker are measured.
25.0 mL of Liquid A are put in one sealed
5 L flask, and 25.0 mL of Liquid B are put
in another sealed 5 L flask. The pressure in
each flask is then slowly lowered with a
vacuum pump.
A
A
A
predicted outcome
will be greater than V
will be less than VB
will be equal to
B
It's impossible to predict whether VA or VB will
be greater without more information.
O Eventually both liquids boil, A first and then B.
Eventually both liquids boil, B first and then A.
O Neither liquid will boil
It's impossible to predict whether eitherliquid
boils without more information.
X
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution
Trending nowThis is a popular solution!
Step by stepSolved in 4 steps with 1 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- 11.Why do water molecules tend to stick to each other to form a liquid? A.Because of their bent shape, the oxygen nucleus of one molecule is unshielded and can attract the hydrogen nucleus of another. B.The exposed charge on hydrogen's protons are attracted to unshared electron pairs on oxygen atoms. C.The hydrogen atoms form negative ions that can make ionic bonds with oxygen. D.Water molecules can share electrons with neighboring molecules forming strong covalent bonds.arrow_forward= STATES OF MATTER Understanding consequences of important physical properties ... Liquid A is known to have a higher vapor pressure and higher surface tension than Liquid B. Use these facts to predict the result of each experiment in the table below, if you can. experiment 35.0 mL of Liquid A are put in one sealed 5 L flask, and 35.0 mL of Liquid B are put in another sealed 5 L flask. The pressure in each flask is then slowly lowered with a vacuum pump. 35.0 mL of Liquid A are put in one sealed 5 L flask, and 35.0 mL of Liquid B are put in another sealed 5 L flask. The pressure in each flask is slowly increased by pumping in argon gas. O predicted outcome Eventually both liquids boil, A first and then B. Eventually both liquids boil, B first and then A. Neither liquid will boil It's impossible to predict whether eitherliquid boils without more information. Eventually both liquids boil, A first and then B. Eventually both liquids boil, B first and then A. Neither liquid will boil It's…arrow_forwardThe pressure above a pure sample of solid Substance X at -186. °C is lowered. At what pressure will the sample sublime? Use the phase diagram of X below to find your answer. pressure (atm) 0 atm بات solid 100 liquid 200 x gas temperature (K) Note: your answer must be within 2 atm of the exact answer to be graded correct. 300arrow_forward
- A scientist is comparing the properties of the molecules HF and HCl. Unfortunately, he mixes up the beakers containing the molecules. To solve this, he decides to find the boiling point of each. He finds that the molecule in beaker A has a higher boiling point than beaker B. Which molecule is in beaker A and which is in beaker B? Explain your answer. ***Be sure to include the type of intermolecular forces experienced by BOTH molecules when explaining your answer.***arrow_forwardThe chemical analysis of a water indicates the presence of cations in the following concentrations: Na+ 53 mg/L Mg2+36 mg/L K+ 72 mg/L Fe2+ 98 mg/L Mn2+15 mg/L A local softening company advertises that its softening unit has a capacity of 1000 meq. If water is used at the rate of 15 m³ per day, how frequently (i.e. how many times) will the unit have to be regenerated to provide the householder with soft water? (Na = 23, Mg = 24.4, K = 39, Fe = 55.85, Mn = 54.94 gms/mole) Answer: 105 Checkarrow_forwardConsider the structures of dimethyl ether and ethanol. Which statements explain that the normal boiling point of dimethyl ether is 250 K, whereas the normal boiling point of ethanol is 351 K? a b Od H I. dimethyl ether has a lower boiling point because it has hydrogen bonding as its predominant IMF II. more energy is needed to overcome the stronger IMF of ethanol III. ethanol has more atoms, so it has stronger London dispersion forces than dimethyl ether I only I and II only ll only II and III only H-C-0-C-H H H Dimethyl Ether HH II H-C-C-O-H | | HH Ethanolarrow_forward
- The pressure above a pure sample of solid Substance X at - 72. °C is raised. At what pressure will the sample melt? Use the phase diagram of X below to find your answer. 0.8- Hiquid 0.4- solid gas 0. 200 400 600 temperature (K) Note: your answer must be within 0.05 atm of the exact answer to be graded correct. atm pressure (atm)arrow_forwardEssential oils are concentrated liquid containing metabolites extracted from plant sources and are widely used in the production of perfumes. The structures of some of these compounds present in essential oils are presented below. H₂C H₂C H₂C CH3 CH₂ CH₂ Compound A MM: 136.228 g/mol H₂C CH₂ Compound B MM: 168.270 g/mol 1. Arrange the given compounds in terms of increasing boiling points. HC HC H₂C CH3 CH3 OH Compound C MM: 150.212 g/molarrow_forwardA pure solid sample of Substance X is put into an evacuated flask. The flask is heated at a steady rate and the temperature recorded as time passes. Here is a graph of the results: temperature (°C) 180.- 160.- 140.- 120- 100. 80.- 60.- 40. 10. What is the melting point of X? heat added (kJ/mol) Use this graph to answer the following questions: What phase (physical state) of X would you expect to find in the flask after 13 kJ/mol of heat has been added? °C 30. (check all that apply) Osolid O liquid Ogas X 40arrow_forward
- The pressure above a pure sample of solid Substance X at -221. °C is lowered. At what pressure will the sample sublime? Use the phase diagram of X below to find your answer. pressure (atm) 0. atm 0 solid liquid 100 gas 200 temperature (K) Note: your answer must be within 0.25 atm of the exact answer X Śarrow_forwardplease answer quickly thank uarrow_forward19. Which of the following liquids would exhibit the highest vapor pressure at 25.0*C? a. water, boiling point = 100*C b. glycerine, boiling point= 290*C C. ethyl alcohol, boiling point = 78.3*C d. ether, boiling point = 34.6*C band in a cloradarrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY