
Chemistry
9th Edition
ISBN: 9781133611097
Author: Steven S. Zumdahl
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Concept explainers
Question
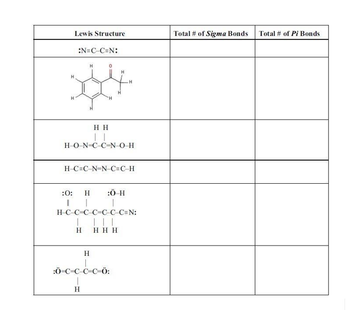
Transcribed Image Text:Lewis Structure
:N=C-C=N:
X4
H-O-N-
:0:
HH
H
H-C=C-N=NC=C-H
CC-NOH
:Ö-H
H
HCC-CC-CC-C=N:
H HHH
H
0-C-C-C-C-Ö:
I
H
Total # of Sigma Bonds
Total # of Pi Bonds
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution
Trending nowThis is a popular solution!
Step by stepSolved in 3 steps with 3 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- A stable triatomic molecule can be formed that contains one atom each of nitrogen, sulfur, and fluorine. Three bonding structures are possible, depending on which is the central atom: NSF, SNF, and SFN. (a) Write a Lewis diagram for each of these molecules, indicating the formal charge on each atom. (b) Often, the structure with the least separation of formal charge is the most stable. Is this statement consistent with the observed structure for this molecule—namely, NSF, which has a central sulfur atom? (c) Does consideration of the electronegativities of N, S, and F from Figure 3.18 help rationalize this observed structure? Explain.arrow_forwardWrite Lewis structures for the following: (a) H2 (b) HBr (c) PCl3 (d) SF2 (e) H2CCH2 (f) HNNH (g) H2CNH (h) NO (i) N2 (j) CO (k) CNarrow_forwardThe percent ionic character of a bond can be approximated by the formula 16+3.52 , where is the magnitude of the difference in the electronegativities of the atoms (see Fig. 3.18). Calculate the percent ionic character of HF, HCl, HBr, HI, and CsF, and compare the results with those in Table 3.7.arrow_forward
- Use formal charge to choose the best Lewis structure for CH3SOCH3. H :Ö H H :ö H B) H-C=S-ċ-H А) Н-С-$-С-н H H H H H :ö: H C) H-C=Š-Ċ-H H :ö: H D) H-C-S-Ċ-H H H A В O Darrow_forward9. Use formal charge to choose the best Lewis structure for CH;SOCH3. H :ö: H н :о н a) H-C=S- ç-H b) H-C-S-C-H H H H H :0: H H :0: H c) H-C-S-C-H d) H-C=s-C-H H H H Harrow_forwardWhich one of the following pairs of Lewis structures represents resonance? 1. :0 C=c II. H H-C-c c=0: Hi C=0: H :O=C-ö: III. :0=C=0:arrow_forward
- Choose the best Lewis structure for OCI 2. O:C=Ö-C: O:ä-ö=cl: Od=0=c!: OCI=o=cl: O:ä-ö-ä:arrow_forwardWhich of the following pairs are NOT resonance structures? + H₂C-0-N=0: and H₂C-O=N-0: :0=c=0: =Ö: and :Ö= H₂C-0-N=0: and H₂C- Each of these pairs represents resonance structures. O None of these pairs represents resonance structures.arrow_forwardA molecule is connected H₂CC (O) CH₂ where the oxygen is connected to the middle carbon and the outside carbons are also connected to the middle carbon. The hydrogens are connected to the outside carbons. Which of the following represents the Lewis Dot Structure for this compound? A) B) H-C-C-C-H C) H H OB H DE OD DA DC H H H 80: H H H-C-C-C-H HC- H-C-C=C-H Both A and B are correct H o: H Both C and D are correct H 80: H D) H ●● H-C-C-C-H H E) H H H - 80: H ●● H -C-C-H 80: Harrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you

 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning Chemistry: An Atoms First ApproachChemistryISBN:9781305079243Author:Steven S. Zumdahl, Susan A. ZumdahlPublisher:Cengage Learning
Chemistry: An Atoms First ApproachChemistryISBN:9781305079243Author:Steven S. Zumdahl, Susan A. ZumdahlPublisher:Cengage Learning Principles of Modern ChemistryChemistryISBN:9781305079113Author:David W. Oxtoby, H. Pat Gillis, Laurie J. ButlerPublisher:Cengage Learning
Principles of Modern ChemistryChemistryISBN:9781305079113Author:David W. Oxtoby, H. Pat Gillis, Laurie J. ButlerPublisher:Cengage Learning Chemistry by OpenStax (2015-05-04)ChemistryISBN:9781938168390Author:Klaus Theopold, Richard H Langley, Paul Flowers, William R. Robinson, Mark BlaserPublisher:OpenStax
Chemistry by OpenStax (2015-05-04)ChemistryISBN:9781938168390Author:Klaus Theopold, Richard H Langley, Paul Flowers, William R. Robinson, Mark BlaserPublisher:OpenStax Introductory Chemistry: A FoundationChemistryISBN:9781337399425Author:Steven S. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
Introductory Chemistry: A FoundationChemistryISBN:9781337399425Author:Steven S. Zumdahl, Donald J. DeCostePublisher:Cengage Learning


Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry: An Atoms First Approach
Chemistry
ISBN:9781305079243
Author:Steven S. Zumdahl, Susan A. Zumdahl
Publisher:Cengage Learning

Principles of Modern Chemistry
Chemistry
ISBN:9781305079113
Author:David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Publisher:Cengage Learning

Chemistry by OpenStax (2015-05-04)
Chemistry
ISBN:9781938168390
Author:Klaus Theopold, Richard H Langley, Paul Flowers, William R. Robinson, Mark Blaser
Publisher:OpenStax

Introductory Chemistry: A Foundation
Chemistry
ISBN:9781337399425
Author:Steven S. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning