
Chemistry
10th Edition
ISBN: 9781305957404
Author: Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Question
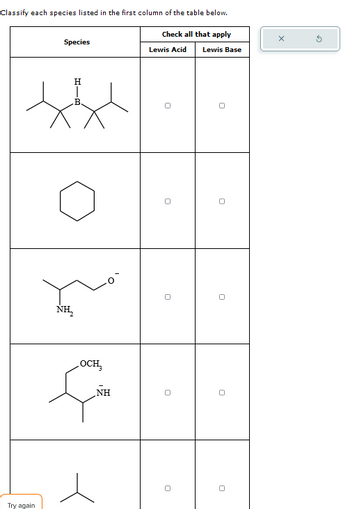
Transcribed Image Text:Lewis Base
Classify each species listed in the first column of the table below.
Check all that apply
Species
Н
В
HIB
Lewis Acid
NH₂
OCH₂
NH
Try again
n
n
U
U
0
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by stepSolved in 2 steps with 2 images

Knowledge Booster
Similar questions
- Classify cach speces Iisted in the first column of the table below. Check all that apply Species Lewis Acid Lewis Base NH но NH (CH;CH,) NH CH но CH, NHarrow_forwardComplete the table below. Round each of your entries to 2 significant digits. You may assume the temperature is 25 °C. conjugate acid conjugate base x10 formula K. formula H,CO3 -7 4.5 X 10 C,H,N - 9 1.7 X 10 HSO, 0.012 Continue O 2021 McGraw HilLLIG AILRights Reserved.arrow_forwardRank the following acids from strongest to weakest CI F Br OH ОН ОН no son no you you H3C H3C о 1 2 3 1>2>3 1>3>2 2>1>3 2>3>1 3>1>2 3>2>1arrow_forward
- List the following bases in order of strength, strongest to weakest. O NH4OH, Kb=1.8x10-3 O NH3, Kb=1.8x10-5 H2NOH, Kµ=1.2x10-8 O CH3NH2, Kp=4.2x10-4 O Pb(OH)2, Kb=9.6x10-4 Submit Answer Tries 0/99 This discussion is closed. Send Feedbackarrow_forwardOTfset%3Dnext&assignmentProblemlD=D18arrow_forwardUse the table below to order the following from the strongest to the weakest acid. Formula Value of Ka HF 7.2 × 10-4 HOCI -8 HOCI 3.5 × 107 From the strongest to the weakest acid Drag and drop your selection from the following list to complete the answer: H₂O HC1 HFarrow_forward
- [Review Topics] [References] Use the References to access important values if needed for this question. 1. The formula for the conjugate base of HNO2 is 2. The formula for the conjugate acid of CH3COO is Submit Answer Retry Entire Group 1 more group attempt remaining SONY WEB DXX DISPLA OFF 0 Next 8:37 PM 4/29/2022arrow_forward3 parts to this questionarrow_forwardDraw the conjugate base for the acid (CH,),C=OH*. Remember to include charges and non-bonding electrons where necessary. Erase Draw Rings More Selectarrow_forward
- 15 16 17 18 19 20 21 22 23 24 25 26 27 ) Fill in the missing chemical formulae in the tables below. acid conjugate base base conjugate acid do H,0 H,0 H,O HPO нсо, NH, Submit Assignment Continue MacBook Air F12 ►► F10 FII F9 F8 000 O00 F4 F7 80 F3 F6 F5 esc F1 & @ # $ 9 7 8 3 4 5 7arrow_forwardClassify species listed in the first column of the table below. Species NH (CH,CH,),NH 2 N Check all that apply Lewis Acid O O O O O Lewis Base O O O O O X olo Ararrow_forwardO ACIDS AND BASES Calculating the pH of a weak acid solution The acid dissociation constant K of alloxanic acid (HCH,N,0,) is 2.24 x 10. Calculate the pH of a 2.8M solution of alloxanic acid. Round your answer to 1 decimal place. pH = Check Explanation O 2021 McGraw-Hill Education. All Rights Re APRarrow_forward
arrow_back_ios
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY