
Chemistry
10th Edition
ISBN: 9781305957404
Author: Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Concept explainers
Question
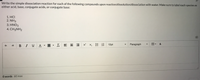
Transcribed Image Text:Write the simple dissociation reaction for each of the following compounds upon reaction/dissolution/dissociation with water. Make sure to label each species as
either acid, base, conjugate acids, or conjugate base:
1. HCI
2. NH3
3. HNO3
4. CH3NH2
BIU
A
I E
12pt
- Paragraph
fr
A
O words 60 max
Expert Solution
arrow_forward
Step 1
Since your question has multiple sub-parts, we will answer the first three sub-parts for you. If you want residual sub-parts to be answered, then please resubmit the entire question and mention those sub-parts you want us to answer.
An acid is a compound that gives the hydrogen ions in the solution. Base accepts a proton in the solution, also they give hydroxide ion in solution.
Step by stepSolved in 2 steps

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- 19.) Rank the following compounds below from most acidic to least acidic. H NH2 H. H. NH2 Molecule A Molecule B Molecule C Molecule D A. B>D> A > C В. А>С>D > B С. А>С>В > D D. C>A> D > B E. C>A> B > Darrow_forwardSelect every substance that is either a conjugate acid or a conjugate base for ammonia (NH3). Question 7 options: NaOH HCl NH2 NH2+ NH2- NH3+ NH4+ NH2NH2 NH3NH3 N2arrow_forwardIt is possible for a compound to function as both an acid and a base. True False Every compound is either an acid or a base. True Falsearrow_forward
- Identify the strongest acidO H2TeO H2SO H2SeO H2OO not enough information is availablearrow_forwardPlease don't provide handwritten solutionarrow_forwardChoose the pair that does not constitute a conjugate acid-base pair. A. CgH;CO2H / C6H;CO2 B. H30* / H20 O C. H;PO4 / PO,3- D. H2SO3 / HSO3arrow_forward
- In a triprotic acid, which Ka has the highest value? Select one: O a. Ka1 O b. Ka2 О с. Каз O d. Kb1 e. Кр2arrow_forwardplease explain each question in details. thanksarrow_forwardFor the system below, if the position of the equilibrium lies to the LEFT. Which is the strongest acid in the system? CH3NH2 + NH3OH+ ⇌ NH2OH + CH3NH3+ Question options: CH3NH3+ CH3NH2 CH3NH2 and NH3OH+ are equally strong acids NH3OH+ NH2OHarrow_forward
- Identify the correct conjugate acid-base pairs BrO 1. HF 2. HCIO2 CIO2 3. HCN 4. HBRO CN MacBook Air 80 888 DII 26 F3 F4 F8 17 %23 2$ & *- 3 4. 6 7 R Y D F Garrow_forwardUsing the Brønsted theory, classify the compounds as either an acid or a base. C,H,OH CH,CH,O Acid CH,CH,NH Answer Bank Basearrow_forwardIdentify the weaker acid in each of the following groups. I: HIO3, HIO2 Il: H20, H2S II: HBRO, HCIO HIO3 H2S, HCIO HIO2 H2S, HBRO HIO2 H20, HBro HIO3 H20, HBroarrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY