
Chemistry
10th Edition
ISBN: 9781305957404
Author: Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Concept explainers
Question
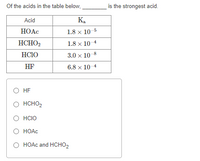
Transcribed Image Text:Of the acids in the table below,
is the strongest acid.
Acid
Ka
HOẠC
1.8 x 10-5
HCHO2
1.8 x 10 4
HC1O
3.0 x 10 8
HF
6.8 x 10 4
O HF
O HCHO,
O HCIO
O HOAC
O HOAC and HCHO2
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution
Trending nowThis is a popular solution!
Step by stepSolved in 2 steps with 2 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- Among the following oxoacids, the correct decreasing order of acid strength is: O HOCI>HCI02>HC103 > HC104 HCI02> HC103 > HCI04> HOCI HCI04> HC103 > HCI02> HOCI HCIO > HCIO3 > HCI04> HOCI2 Oarrow_forwardAnswer all questions.arrow_forwardA strong acid has a weak bond to its acidic proton, where as a weak acid has a strong bond to its acidic proton. Explain.arrow_forward
- SOLVE PLEASE Using Lewis theory, say who is the acid, who is the base, write the structure of the product. Perform the electronic configuration, the Lewis structure of the reactants AI BY3 + PH3 = Write the products and say who the conjugate pair is. HS + HCO3arrow_forwardSolve correctly please with explanation need.arrow_forwardWhich do you expect to be the stronger base: HCN or HNC? Explain. Hint: Draw out the complete Lewis structure for each molecule.arrow_forward
- Show the products of this acid-base reaction and predict whether the equilibrium favors the reactants or the products. Use curved arrows. the acid is listed first. Is it reactant favored or product favored?arrow_forwardGive clear answerarrow_forwardCan someone please show how I would draw the lewis structures for both of these compunds? The orginal question was which compound is more acidic, I understand the compound on the right is more acidic because it holds an sp2 bond, while the compound on th right holds an sp3 bond, but that is rally hard to see since i dont know how to draw out that lewis structure. and am i looking at the O atom to decide which is more acidic, thank you so much in advance + CH3OCH 3 H or H3C OH CH3arrow_forward
arrow_back_ios
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY