
Biochemistry
9th Edition
ISBN: 9781319114671
Author: Lubert Stryer, Jeremy M. Berg, John L. Tymoczko, Gregory J. Gatto Jr.
Publisher: W. H. Freeman
expand_more
expand_more
format_list_bulleted
Question
Match each component of the reaction in the figure with the corresponding relative energy level.
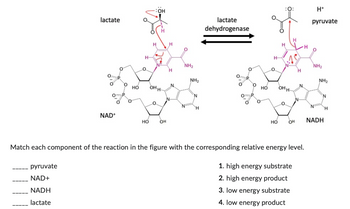
Transcribed Image Text:lactate
pyruvate
NAD+
NADH
lactate
NAD+
HO
H-
0.
HO
H
OH
OH H-
OH
NH₂
NH₂
N
H
lactate
dehydrogenase
O
HO
H-
HO
:0:
H
OH H-
H
OH
H
Match each component of the reaction in the figure with the corresponding relative energy level.
1. high energy substrate
2. high energy product
3. low energy substrate
4. low energy product
H+
pyruvate
NH₂
N
NH₂
NADH
H
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by stepSolved in 3 steps with 1 images

Knowledge Booster
Similar questions
- In the reaction below, the products have a higher free energy (G) than the reactants. What can you conclude about this reaction? AB + C A) It is endergonic with a negative delta G. 4 B) It is exergonic with a negative delta G. AC + B C) It is endergonic with a positive delta G. E) A and D D) It will proceed more quickly in the presence of a catalyst. F) B and D G) C and Darrow_forwardThe first and second laws of thermodynamics are useful for biochemists who investigate chemical reactions in living organisms. Explain why the third law is not useful.arrow_forwardWhat amount of catalyst is consumed in the reaction it catalyzes?arrow_forward
- Entropy increase A) with every reaction B) only in open systems C) only under certain reaction conditions D) only in the physical world. This cannot happen in cells otherwise cells would lose their needed energyarrow_forwardIdentify the letter (A, B, C) that indicates the change in the energy of the entire reaction according to this energy profile. Explain why you choose the particular energy change. Include in your answer information about the ones you did not chose. alyzed Reaction Energy Exothermic Reaction Profilearrow_forwardwhat is free Gibbs energy and write the expression of free energy change. Define the exergonic and endergonic processes.arrow_forward
- if the reaction given below occurs and pure A and B were mixed, which of the following would take place as equilibrium was established A + B ⇌ C a. the concentration of C would increase for a time, then remain constant b. the concentration of A would increase for a time, then decrease c. the concentration of B would increase for a time, then remain constantarrow_forwardWhat energy requirements must be met in order for a reaction to be favorable?arrow_forwardLabel the following statements true or false: (a) A reaction is said to be spontaneous when it can proceed in either the forward or reverse direction. (b) A spontaneous process always happens very quickly.arrow_forward
- The concept of determining which reactant is limiting and which is in excess is akin to determining the number of sandwiches that can be made from a set number of ingredients. Assuming that a cheese sandwich consists of 2 slices of bread and 3 slices of cheese, determine the number of whole cheese sandwiches that can be prepared from 36 slices of bread and 51 slices of cheese.arrow_forwardIn the lab, a group of students is carrying out the reaction described and graphed in the previous question, but it is not proceeding. One student suggests heating it up to make it proceed more rapidly. Another student suggests adding an enzyme to the reaction instead. They try both and find that heating speeds up the reaction, and adding an enzyme also speeds up the reaction. A) How does heating the reaction in a test tube cause the reaction to occur at a higher rate? B) How does adding an enzyme enable the reaction to occur at a faster rate?arrow_forwardWhat is expected to happen to the reaction rate with increasing temperature? (Enzyme kinetics)arrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 BiochemistryBiochemistryISBN:9781319114671Author:Lubert Stryer, Jeremy M. Berg, John L. Tymoczko, Gregory J. Gatto Jr.Publisher:W. H. Freeman
BiochemistryBiochemistryISBN:9781319114671Author:Lubert Stryer, Jeremy M. Berg, John L. Tymoczko, Gregory J. Gatto Jr.Publisher:W. H. Freeman Lehninger Principles of BiochemistryBiochemistryISBN:9781464126116Author:David L. Nelson, Michael M. CoxPublisher:W. H. Freeman
Lehninger Principles of BiochemistryBiochemistryISBN:9781464126116Author:David L. Nelson, Michael M. CoxPublisher:W. H. Freeman Fundamentals of Biochemistry: Life at the Molecul...BiochemistryISBN:9781118918401Author:Donald Voet, Judith G. Voet, Charlotte W. PrattPublisher:WILEY
Fundamentals of Biochemistry: Life at the Molecul...BiochemistryISBN:9781118918401Author:Donald Voet, Judith G. Voet, Charlotte W. PrattPublisher:WILEY BiochemistryBiochemistryISBN:9781305961135Author:Mary K. Campbell, Shawn O. Farrell, Owen M. McDougalPublisher:Cengage Learning
BiochemistryBiochemistryISBN:9781305961135Author:Mary K. Campbell, Shawn O. Farrell, Owen M. McDougalPublisher:Cengage Learning BiochemistryBiochemistryISBN:9781305577206Author:Reginald H. Garrett, Charles M. GrishamPublisher:Cengage Learning
BiochemistryBiochemistryISBN:9781305577206Author:Reginald H. Garrett, Charles M. GrishamPublisher:Cengage Learning Fundamentals of General, Organic, and Biological ...BiochemistryISBN:9780134015187Author:John E. McMurry, David S. Ballantine, Carl A. Hoeger, Virginia E. PetersonPublisher:PEARSON
Fundamentals of General, Organic, and Biological ...BiochemistryISBN:9780134015187Author:John E. McMurry, David S. Ballantine, Carl A. Hoeger, Virginia E. PetersonPublisher:PEARSON

Biochemistry
Biochemistry
ISBN:9781319114671
Author:Lubert Stryer, Jeremy M. Berg, John L. Tymoczko, Gregory J. Gatto Jr.
Publisher:W. H. Freeman

Lehninger Principles of Biochemistry
Biochemistry
ISBN:9781464126116
Author:David L. Nelson, Michael M. Cox
Publisher:W. H. Freeman

Fundamentals of Biochemistry: Life at the Molecul...
Biochemistry
ISBN:9781118918401
Author:Donald Voet, Judith G. Voet, Charlotte W. Pratt
Publisher:WILEY

Biochemistry
Biochemistry
ISBN:9781305961135
Author:Mary K. Campbell, Shawn O. Farrell, Owen M. McDougal
Publisher:Cengage Learning

Biochemistry
Biochemistry
ISBN:9781305577206
Author:Reginald H. Garrett, Charles M. Grisham
Publisher:Cengage Learning

Fundamentals of General, Organic, and Biological ...
Biochemistry
ISBN:9780134015187
Author:John E. McMurry, David S. Ballantine, Carl A. Hoeger, Virginia E. Peterson
Publisher:PEARSON