
Introduction to Chemical Engineering Thermodynamics
8th Edition
ISBN: 9781259696527
Author: J.M. Smith Termodinamica en ingenieria quimica, Hendrick C Van Ness, Michael Abbott, Mark Swihart
Publisher: McGraw-Hill Education
expand_more
expand_more
format_list_bulleted
Question
Help with the following question please answer all parts.
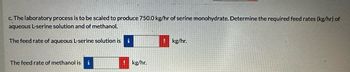
Transcribed Image Text:c. The laboratory process is to be scaled to produce 750.0 kg/hr of serine monohydrate. Determine the required feed rates (kg/hr) of
aqueous L-serine solution and of methanol.
The feed rate of aqueous L-serine solution is
i
! kg/hr.
The feed rate of methanol is i
kg/hr.
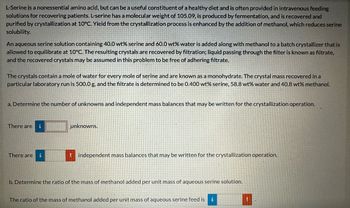
Transcribed Image Text:L-Serine is a nonessential amino acid, but can be a useful constituent of a healthy diet and is often provided in intravenous feeding
solutions for recovering patients. L-serine has a molecular weight of 105.09, is produced by fermentation, and is recovered and
purified by crystallization at 10°C. Yield from the crystallization process is enhanced by the addition of methanol, which reduces serine
solubility.
An aqueous serine solution containing 40.0 wt% serine and 60.0 wt% water is added along with methanol to a batch crystallizer that is
allowed to equilibrate at 10°C. The resulting crystals are recovered by filtration; liquid passing through the filter is known as filtrate,
and the recovered crystals may be assumed in this problem to be free of adhering filtrate.
The crystals contain a mole of water for every mole of serine and are known as a monohydrate. The crystal mass recovered in a
particular laboratory run is 500.0 g, and the filtrate is determined to be 0.400 wt% serine, 58.8 wt% water and 40.8 wt% methanol.
a. Determine the number of unknowns and independent mass balances that may be written for the crystallization operation.
There are
i
There are i
unknowns.
!independent mass balances that may be written for the crystallization operation.
b. Determine the ratio of the mass of methanol added per unit mass of aqueous serine solution.
The ratio of the mass of methanol added per unit mass of aqueous serine feed is i
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by stepSolved in 2 steps with 3 images

Knowledge Booster
Similar questions
Recommended textbooks for you
 Introduction to Chemical Engineering Thermodynami...Chemical EngineeringISBN:9781259696527Author:J.M. Smith Termodinamica en ingenieria quimica, Hendrick C Van Ness, Michael Abbott, Mark SwihartPublisher:McGraw-Hill Education
Introduction to Chemical Engineering Thermodynami...Chemical EngineeringISBN:9781259696527Author:J.M. Smith Termodinamica en ingenieria quimica, Hendrick C Van Ness, Michael Abbott, Mark SwihartPublisher:McGraw-Hill Education Elementary Principles of Chemical Processes, Bind...Chemical EngineeringISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...Chemical EngineeringISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY Elements of Chemical Reaction Engineering (5th Ed...Chemical EngineeringISBN:9780133887518Author:H. Scott FoglerPublisher:Prentice Hall
Elements of Chemical Reaction Engineering (5th Ed...Chemical EngineeringISBN:9780133887518Author:H. Scott FoglerPublisher:Prentice Hall
 Industrial Plastics: Theory and ApplicationsChemical EngineeringISBN:9781285061238Author:Lokensgard, ErikPublisher:Delmar Cengage Learning
Industrial Plastics: Theory and ApplicationsChemical EngineeringISBN:9781285061238Author:Lokensgard, ErikPublisher:Delmar Cengage Learning Unit Operations of Chemical EngineeringChemical EngineeringISBN:9780072848236Author:Warren McCabe, Julian C. Smith, Peter HarriottPublisher:McGraw-Hill Companies, The
Unit Operations of Chemical EngineeringChemical EngineeringISBN:9780072848236Author:Warren McCabe, Julian C. Smith, Peter HarriottPublisher:McGraw-Hill Companies, The

Introduction to Chemical Engineering Thermodynami...
Chemical Engineering
ISBN:9781259696527
Author:J.M. Smith Termodinamica en ingenieria quimica, Hendrick C Van Ness, Michael Abbott, Mark Swihart
Publisher:McGraw-Hill Education

Elementary Principles of Chemical Processes, Bind...
Chemical Engineering
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY

Elements of Chemical Reaction Engineering (5th Ed...
Chemical Engineering
ISBN:9780133887518
Author:H. Scott Fogler
Publisher:Prentice Hall


Industrial Plastics: Theory and Applications
Chemical Engineering
ISBN:9781285061238
Author:Lokensgard, Erik
Publisher:Delmar Cengage Learning

Unit Operations of Chemical Engineering
Chemical Engineering
ISBN:9780072848236
Author:Warren McCabe, Julian C. Smith, Peter Harriott
Publisher:McGraw-Hill Companies, The