
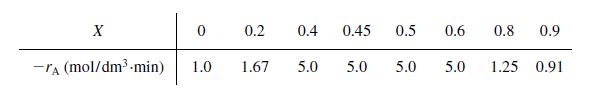
The exothermic reaction of stillbene (A) to form the economically important trospophene (B) and methane (C), i.e.,
A ⎯⎯→ B +C
was carried out adiabatically and the following data recorded:
The entering molar flow rate of A was 300 mol/min.
(a)What are the PFR and CSTR volumes necessary to achieve 40% conversion? (VPFR=72 dm3,VCSTR=24 dm3)
(b)Over what range of conversions would the CSTR and PFR reactor volumes be identical?
(c)What is the maximum conversion that can be achieved in a 105-dm3
CSTR?
(d)What conversion can be achieved if a 72-dm3PFR is followed in series by a 24-dm3CSTR?
(e)What conversion can be achieved if a 24-dm3CSTR is followed in a series by a 72-dm3PFR?
(f)Plot the conversion and rate of reaction as a function of PFR reactor volume up to a volume of100dm3.
Trending nowThis is a popular solution!
Step by stepSolved in 9 steps with 12 images

can youdo part d and e now
can youdo part d and e now
- A substance (A) reacts to form another substance (B) according to: 3B(g) = 2A(g) The reaction is run at a particular temperature with the concentrations of A and B monitored over time and plotted on the graph below. At what time was equilibrium fırst reached and what is the approximate value of the equilibrium constant? 2.0 1.8 E 1.6 1.4 [B] 1.2 1.0 0.8 0.6 0.4 [A] 0.2 0.0 10 20 30 40 50 60 70 80 90 100 110 120 130 140 150 time / min O Equilibrium was reached at 70 min with K. = 0.0056. O Equilibrium was reached at 30 min with K. = 0.0056. Equilibrium was reached at 30 min with K. = 0.023. Equilibrium was reached at 70 min with K. = 0.023. concentration / Marrow_forwardReactant A decomposes as follows: A Rdesired S T U where n, are reaction orders. Qualitatively find what flow (plug, mixed, or inter- mediate) and what CAO (high, low, or intermediate) should be used for a high conversion to R. 11 12 из Па th Case (a) 1 1 1 1 (b) 1 2 1 1 (c) 2 1 1 1 (d) 1 2 (e) 1 (f) 1 02 21 1 0 2 1 1arrow_forwardThe decomposition of formic acid vapor according to the following reaction at 550oC follows a first-order kinetics, and at the above temperature, the reaction has a half-life of 240 s. HCO2H (g) ® CO2(g) + H2(g); Rate = k[HCO2H] If the initial concentration of HCO2H was 0.160 M, what is its concentration after four half-lives? (A) 0.010 M (B) 0.020 M (C) 0.040 M (D) 0.0050 Marrow_forward
- Solve correctly please AsAp.arrow_forwardOver the past several years, the atmospheric ozone concentration over Antarctica has decreased during the winter. The reaction that causes this is shown below:NO(g)+O3(g)⟶NO2(g)+O2(g)Using the data obtained at 100 degree Celsius, determine the rate law for this process: Trial [NONO] [O3O3 ] Rate 1 1.00x10−61.00x10^-6 3.00x10−63.00x10^-6 6.60x10−56.60x10^-5 2 1.00x10−61.00x10^-6 6.00x10−66.00x10^-6 1.32x10−41.32x10^-4 3 1.00x10−61.00x10^-6 9.00x10−69.00x10^-6 1.98x10−41.98x10^-4 4 3.00x10−63.00x10^-6 9.00x10−69.00x10^-6 1.78x10−31.78x10^-3 None of these. rate=k[NO] rate=k[NO][O3] rate=k[NO]^2[O3] rate=k[NO][O3]^2arrow_forwardIn a batch reactor, a substance A was processed, which generated different products (D and U), through competitive parallel reactions with the following reaction kinetics: After 20 minutes of reaction, it was determined that the composition of the reaction medium was CA = 1 mol/L, CD = 5 mol/L, CU = 2 mol/L. The option that indicates, respectively, the instantaneous and global selectivities at the end of the reaction are: A) 3 and 2. B) 2.5 and 2. C) 2 and 3. D) 2 and 2.5.arrow_forward
 Introduction to Chemical Engineering Thermodynami...Chemical EngineeringISBN:9781259696527Author:J.M. Smith Termodinamica en ingenieria quimica, Hendrick C Van Ness, Michael Abbott, Mark SwihartPublisher:McGraw-Hill Education
Introduction to Chemical Engineering Thermodynami...Chemical EngineeringISBN:9781259696527Author:J.M. Smith Termodinamica en ingenieria quimica, Hendrick C Van Ness, Michael Abbott, Mark SwihartPublisher:McGraw-Hill Education Elementary Principles of Chemical Processes, Bind...Chemical EngineeringISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...Chemical EngineeringISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY Elements of Chemical Reaction Engineering (5th Ed...Chemical EngineeringISBN:9780133887518Author:H. Scott FoglerPublisher:Prentice Hall
Elements of Chemical Reaction Engineering (5th Ed...Chemical EngineeringISBN:9780133887518Author:H. Scott FoglerPublisher:Prentice Hall
 Industrial Plastics: Theory and ApplicationsChemical EngineeringISBN:9781285061238Author:Lokensgard, ErikPublisher:Delmar Cengage Learning
Industrial Plastics: Theory and ApplicationsChemical EngineeringISBN:9781285061238Author:Lokensgard, ErikPublisher:Delmar Cengage Learning Unit Operations of Chemical EngineeringChemical EngineeringISBN:9780072848236Author:Warren McCabe, Julian C. Smith, Peter HarriottPublisher:McGraw-Hill Companies, The
Unit Operations of Chemical EngineeringChemical EngineeringISBN:9780072848236Author:Warren McCabe, Julian C. Smith, Peter HarriottPublisher:McGraw-Hill Companies, The





