
Introduction to Chemical Engineering Thermodynamics
8th Edition
ISBN: 9781259696527
Author: J.M. Smith Termodinamica en ingenieria quimica, Hendrick C Van Ness, Michael Abbott, Mark Swihart
Publisher: McGraw-Hill Education
expand_more
expand_more
format_list_bulleted
Question
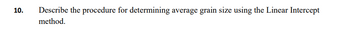
Transcribed Image Text:10.
Describe the procedure for determining average grain size using the Linear Intercept
method.
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by stepSolved in 2 steps

Knowledge Booster
Similar questions
- 2. In 3D printing, small liquid droplets are formed when a liquid jet breaks up in spray and fuel injection processes. The resulting droplet diameter d is thought to depend on liquid density p, viscosity µ, and surface tension o, as well as jet speed V and its diameter D. How to use dimensional analysis to characterize this process (using p, V and D as the repeating parameters)?arrow_forwardCould you possibly draw the graph out? im a bit confused with that partarrow_forwardIn scanning electron microscope SEM, light is sent to the sample in order to get different signals and data from the sample. A) Doğru B Yanlışarrow_forward
- Differentiate the following terms used in Chemical processing Extraction vs Distillationarrow_forwardIn microfiltration, why is an operation that combines constant-flux and constant-pressure operations used?arrow_forward1.a ) If two bituminous samples have the same consistency , describe the criteria which can be used to select the better bituminous sample. b) what does higher softening point of bitumen indicate??arrow_forward
- What are the ideal flow-pattern models?arrow_forwardWhat is the best method for estimating the distribution of nonkey components at the actual (operating) reflux?arrow_forwardUse Graphical representation to show how crystal growth rate depends on crystallization temperature. Label Tm and Tg on the x axis and label the region where you will find large crystals to nucleate and growarrow_forward
- 4. Calculate the theoretical (adiabatic) flame temperature for CO burned at constant pressure with 100% excess air, when the reactants enter at 100°C. CO + 1/2 O2 = CO%@)arrow_forwardSolve the percent error columnarrow_forwardwhat are the parameters that vary along the z axis of membranesm that causes anisotropy?arrow_forward
arrow_back_ios
arrow_forward_ios
Recommended textbooks for you
 Introduction to Chemical Engineering Thermodynami...Chemical EngineeringISBN:9781259696527Author:J.M. Smith Termodinamica en ingenieria quimica, Hendrick C Van Ness, Michael Abbott, Mark SwihartPublisher:McGraw-Hill Education
Introduction to Chemical Engineering Thermodynami...Chemical EngineeringISBN:9781259696527Author:J.M. Smith Termodinamica en ingenieria quimica, Hendrick C Van Ness, Michael Abbott, Mark SwihartPublisher:McGraw-Hill Education Elementary Principles of Chemical Processes, Bind...Chemical EngineeringISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...Chemical EngineeringISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY Elements of Chemical Reaction Engineering (5th Ed...Chemical EngineeringISBN:9780133887518Author:H. Scott FoglerPublisher:Prentice Hall
Elements of Chemical Reaction Engineering (5th Ed...Chemical EngineeringISBN:9780133887518Author:H. Scott FoglerPublisher:Prentice Hall
 Industrial Plastics: Theory and ApplicationsChemical EngineeringISBN:9781285061238Author:Lokensgard, ErikPublisher:Delmar Cengage Learning
Industrial Plastics: Theory and ApplicationsChemical EngineeringISBN:9781285061238Author:Lokensgard, ErikPublisher:Delmar Cengage Learning Unit Operations of Chemical EngineeringChemical EngineeringISBN:9780072848236Author:Warren McCabe, Julian C. Smith, Peter HarriottPublisher:McGraw-Hill Companies, The
Unit Operations of Chemical EngineeringChemical EngineeringISBN:9780072848236Author:Warren McCabe, Julian C. Smith, Peter HarriottPublisher:McGraw-Hill Companies, The

Introduction to Chemical Engineering Thermodynami...
Chemical Engineering
ISBN:9781259696527
Author:J.M. Smith Termodinamica en ingenieria quimica, Hendrick C Van Ness, Michael Abbott, Mark Swihart
Publisher:McGraw-Hill Education

Elementary Principles of Chemical Processes, Bind...
Chemical Engineering
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY

Elements of Chemical Reaction Engineering (5th Ed...
Chemical Engineering
ISBN:9780133887518
Author:H. Scott Fogler
Publisher:Prentice Hall


Industrial Plastics: Theory and Applications
Chemical Engineering
ISBN:9781285061238
Author:Lokensgard, Erik
Publisher:Delmar Cengage Learning

Unit Operations of Chemical Engineering
Chemical Engineering
ISBN:9780072848236
Author:Warren McCabe, Julian C. Smith, Peter Harriott
Publisher:McGraw-Hill Companies, The