
Chemistry: The Molecular Science
5th Edition
ISBN: 9781285199047
Author: John W. Moore, Conrad L. Stanitski
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Question
Transcribed Image Text:ks
Window
Help
achieve.macmillanlearning.com
m Learning
pts
c
? a
Tue Sep 10 11:20
+
Chapter 3 HW - General, Organic, and Biological Chemistry for Health Sciences - Achieve
Resources
Solution
Penalized
? Hint
Submit Answer
stion 37 of 37 >
Attempt 7
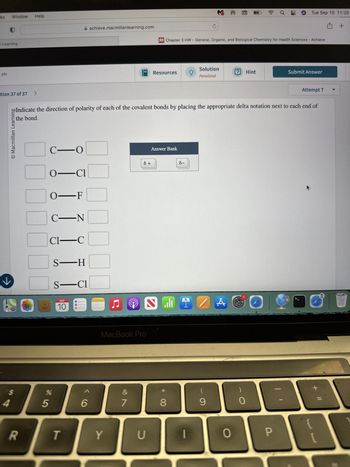
po Indicate the direction of polarity of each of the covalent bonds by placing the appropriate delta notation next to each end of
the bond.
↓
© Macmillan Learning
280
☐ C-O
☐ O-CI
O-F
☐ C_N
Cl―C
S―H
S-C1
SEP
10
☐ ☐ ☐ ☐☐☐☐
30 л
$
%
85
96
R
T
Y
MacBook Pro
&
7
U
8+
Answer Bank
80
8-
I`
ZA
(
)
9
0
O
P
+ 11
A
SAVE
AI-Generated Solution
info
AI-generated content may present inaccurate or offensive content that does not represent bartleby’s views.
Unlock instant AI solutions
Tap the button
to generate a solution
to generate a solution
Click the button to generate
a solution
a solution
Knowledge Booster
Similar questions
- enow.com/ilrn/takeAssignment/takeCovalentActivity.do?locator%3Dassignment-take [References] Acetic acia is responsibie for the sour taste or vihegar. it can be manuracturea using the following reaction: |3| 2 CH3C-OH(g) 2 CH3C-H(g) + O2(g) Use tabulated values of bond energies from the table below to estimate AH for this reaction. Average Bond Energies Single Bonds Bond Bond Energy (kJ/mol) C-C 347 C-H 413 C-O 358 0-H 467 Multiple Bonds Bond Bond Energy (kJ/mol) C=0 1072 C=0 745 O=0 to 495 kJ %3Darrow_forwardRank the elements or compounds in the table below in decreasing order of their boiling points. That is, choose 1 next to the substance with the highest boiling point, choose 2 next to the substance with the next highest boiling point, and so on. substance A B C D chemical symbol, chemical formula or Lewis structure H :0: I | H H-C-C-0-C-H F₂ H :O: ||| : 0: H H 1 .. H H 1 HIC-C-N-C-H H H :NEN 0: boiling point (Choose one) ✓ (Choose one) ✓ (Choose one) (Choose one) Xarrow_forwardcan you please answer question 9.73 and all of the sub problems And show all of the steps to the solutionarrow_forward
- Rank the elements or compounds in the table below in decreasing order of their boiling points. That is, choose 1 next to the substance with the highest boiling point, choose 2 next to the substance with the next highest boiling point, and so on. substance A B U D chemical symbol, chemical formula or Lewis structure H H 1 | H-C C 1 I I H H H Ar H I Ag : 0: C-O-H :0: ||| H-C-C-C-H 44 boiling point ✓ (Choose one) 1 (highest) 2 W N 3 4 (lowest) (Choose one) ↑ (Choose one) (Choose one) ↑arrow_forwardRank the elements or compounds in the table below in decreasing order of their boiling points. That is, choose 1 next to the substance with the highest boiling point, choose 2 next to the substance with the next highest boiling point, and so on. substance A B C D H chemical symbol, chemical formula or Lewis structure H :0: 44 - C- C-H H - H 1 C I Ar H H | | - C - | 1 H H H Ag H CIO : 0: H boiling point ✓ (Choose one) 1 (highest) 2 3 4 (lowest) (Choose one) ✓ (Choose one) ✓arrow_forward10. The following Lewis structures for (a) HCN, (b) C3H;, (c) SnOz, (d) BF3, (e)HOF are incorrect. Explain what is wrong with each one and give a correct structure for the molecule. (Relative positions of atoms are shown correctly.) (a) H-ëN (b) HCC-H (c) 0-Sn-0 (d) :F B :F: (e) H-O-F:arrow_forward
- Rank the elements or compounds in the table below in decreasing order of their boiling points. That is, choose 1 next to the substance with the highest boiling point, choose 2 next to the substance with the next highest boiling point, and so on. substance A B C H H - - chemical symbol, chemical formula or Lewis structure H :0: | || C 1 H | H H | || C-N- | H H - F₂ :NEN 0: :0: - H | C C-O- — H | C I H - H — - H - boiling point (Choose one) ✪ (Choose one) ✪ (Choose one) (Choose one) 09:39 6 00 Aarrow_forwardnk the elements or compounds in the table below in decreasing order of their boiling points. That is, choose 1 next to the substance with the highest boiling nt, choose 2 next to the substance with the next highest boiling point, and so on. substance A B C D chemical symbol, chemical formula or Lewis structure NO :O: H HIC C-H 1 H N₂ :0: || .. HIC N-H 1 H boiling point ✓ (Choose one) 1 (highest) 2 3 4 (lowest) (Choose one) ✓ (Choose one) ✓ X Śarrow_forwardRank the elements or compounds in the table below in decreasing order of their boiling points. That is, choose 1 next to the substance with the highest boiling point, choose 2 next to the substance with the next highest boiling point, and so on. substance A B C D chemical symbol, chemical formula or Lewis structure H :O: H | || | H-C-C-C-H H | | H Ar | | H H Ag H H | | H-C-C-C-O-H 1 H | H boiling point (Choose one) (Choose one) (Choose one) X (Choose one 1 (highest) 2 3 4 (lowest) 5arrow_forward
- A student investigates the physical and chemical properties of various carbon-containing compounds. Thr complete Lewis electron-dot diagrams and boiling points for two compounds, Q and Z, are shown in the following table: B) Any C — H bond in compound Q is shorter than the S — H bond in compound Z. Explain the reason for this difference using principles of atomic structure.arrow_forwardIn each of the four molecules shown below the oxygen atom has two lone pairs of electrons to complete its Octet. H H H i H-C-C-H IT H-C-C-0-H || H HA H B H H H&CH -H HIC OIC-H ннс Η H D Which of these molecules could function as both a hydrogen bond donor and acceptor? OC and D none of the four molecules A and B all four of the molecules Aarrow_forwardRank the elements or compounds in the table below in decreasing order of their boiling points. That is, choose 1 next to the substance with the highest boiling point, choose 2 next to the substance with the next highest boiling point, and so on. substance A B 0 D H chemical symbol, chemical formula or Lewis structure NO :O: H C-C-H I H :0: || H-C-N-H ZIH Н boiling point (Choose one) (Choose one) ✓ (Choose one) ✓ (Choose one) ✓ X Garrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 Chemistry: The Molecular ScienceChemistryISBN:9781285199047Author:John W. Moore, Conrad L. StanitskiPublisher:Cengage Learning
Chemistry: The Molecular ScienceChemistryISBN:9781285199047Author:John W. Moore, Conrad L. StanitskiPublisher:Cengage Learning Chemistry & Chemical ReactivityChemistryISBN:9781133949640Author:John C. Kotz, Paul M. Treichel, John Townsend, David TreichelPublisher:Cengage Learning
Chemistry & Chemical ReactivityChemistryISBN:9781133949640Author:John C. Kotz, Paul M. Treichel, John Townsend, David TreichelPublisher:Cengage Learning Chemistry & Chemical ReactivityChemistryISBN:9781337399074Author:John C. Kotz, Paul M. Treichel, John Townsend, David TreichelPublisher:Cengage Learning
Chemistry & Chemical ReactivityChemistryISBN:9781337399074Author:John C. Kotz, Paul M. Treichel, John Townsend, David TreichelPublisher:Cengage Learning Introductory Chemistry: A FoundationChemistryISBN:9781337399425Author:Steven S. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
Introductory Chemistry: A FoundationChemistryISBN:9781337399425Author:Steven S. Zumdahl, Donald J. DeCostePublisher:Cengage Learning

Chemistry: The Molecular Science
Chemistry
ISBN:9781285199047
Author:John W. Moore, Conrad L. Stanitski
Publisher:Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:9781133949640
Author:John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:9781337399074
Author:John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:Cengage Learning

Introductory Chemistry: A Foundation
Chemistry
ISBN:9781337399425
Author:Steven S. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning