
Pushing Electrons
4th Edition
ISBN: 9781133951889
Author: Weeks, Daniel P.
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Question
help please answer in text form with proper workings and explanation for each and every part and steps with concept and introduction no AI no copy paste remember answer must be in proper format with all working
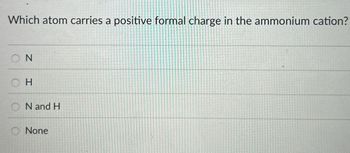
Transcribed Image Text:Which atom carries a positive formal charge in the ammonium cation?
ON
H
N and H
None
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by stepSolved in 2 steps with 1 images

Knowledge Booster
Similar questions
- In an ammonium, nitrogen has a valence of 4, and zero nonbonding electrons. What is the correct formal charge of nitrogen with 4 covalent bonds?arrow_forwardWhich atom bears the formal positive charge in the hydronium ion?arrow_forwardwhat is the complete lewis structure of acetic anhydride such that all atoms have zero formal charge?arrow_forward
- A oxygen with one single bond and three lone pairs is a neutral oxygen an oxonium ion with formal charge -1 an oxyanion ion with formal charge -1 an oxyanion with formal charge +1 an oxonium ion with formal charge +1arrow_forwardThe structure shown below is missing formal charges, but all electrons are shown. What is the formal charge on (A) the oxygen atom (B) the nitrogen atom and (C) the carbon atom that is double-bonded to the nitrogen ? N.arrow_forwardThe oxygen atom in acetone possesses ____ unshared pairs and ____ shared pairs of electrons. The number of electrons that belong to oxygen is ____. Oxygen is a Group ____ element. The formal charge on oxygen in acetone is ____.arrow_forward
- The carbon atom owns one electron from each of shared pairs and two electrons from unshared pair. The number of electrons that belong to carbon is . Carbon is a Group element. Since the carbon atom has one more electron than it would in the neutral, unbonded state, it has a formal charge of –1 . The Lewis structure for the methyl anion is The lithium fragment must have a formal charge ofarrow_forwardEthanol, , is a compound in which the formal charge on all the atoms is zero. Under certain conditions the bond can be broken so that both electrons remain with the oxygen atom. The products are In this structure the oxygen owns one electron from shared pair and two electrons from each of unshared pairs. The total number of electrons belonging to oxygen is Oxygen is a Group element. The formal charge on the oxygen atom is . The correct Lewis structure for the ethoxide ion is Note that the other fragment, the proton, leaves with a formal charge of +1.arrow_forwardDraw the four resonance structures with formal chargearrow_forward
- What atom will have a formal charge in the following structure? CH O Atom A O Atom B Atom C O Atom Darrow_forwardDo any of the atoms in the above compounds have a formal charge?arrow_forwardDraw the best Lewis structure for the linear C ion, including all lone pair electrons and non-zero formal charges.arrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you


 Organic Chemistry: A Guided InquiryChemistryISBN:9780618974122Author:Andrei StraumanisPublisher:Cengage Learning
Organic Chemistry: A Guided InquiryChemistryISBN:9780618974122Author:Andrei StraumanisPublisher:Cengage Learning



Organic Chemistry: A Guided Inquiry
Chemistry
ISBN:9780618974122
Author:Andrei Straumanis
Publisher:Cengage Learning