
Chemistry
10th Edition
ISBN: 9781305957404
Author: Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Question
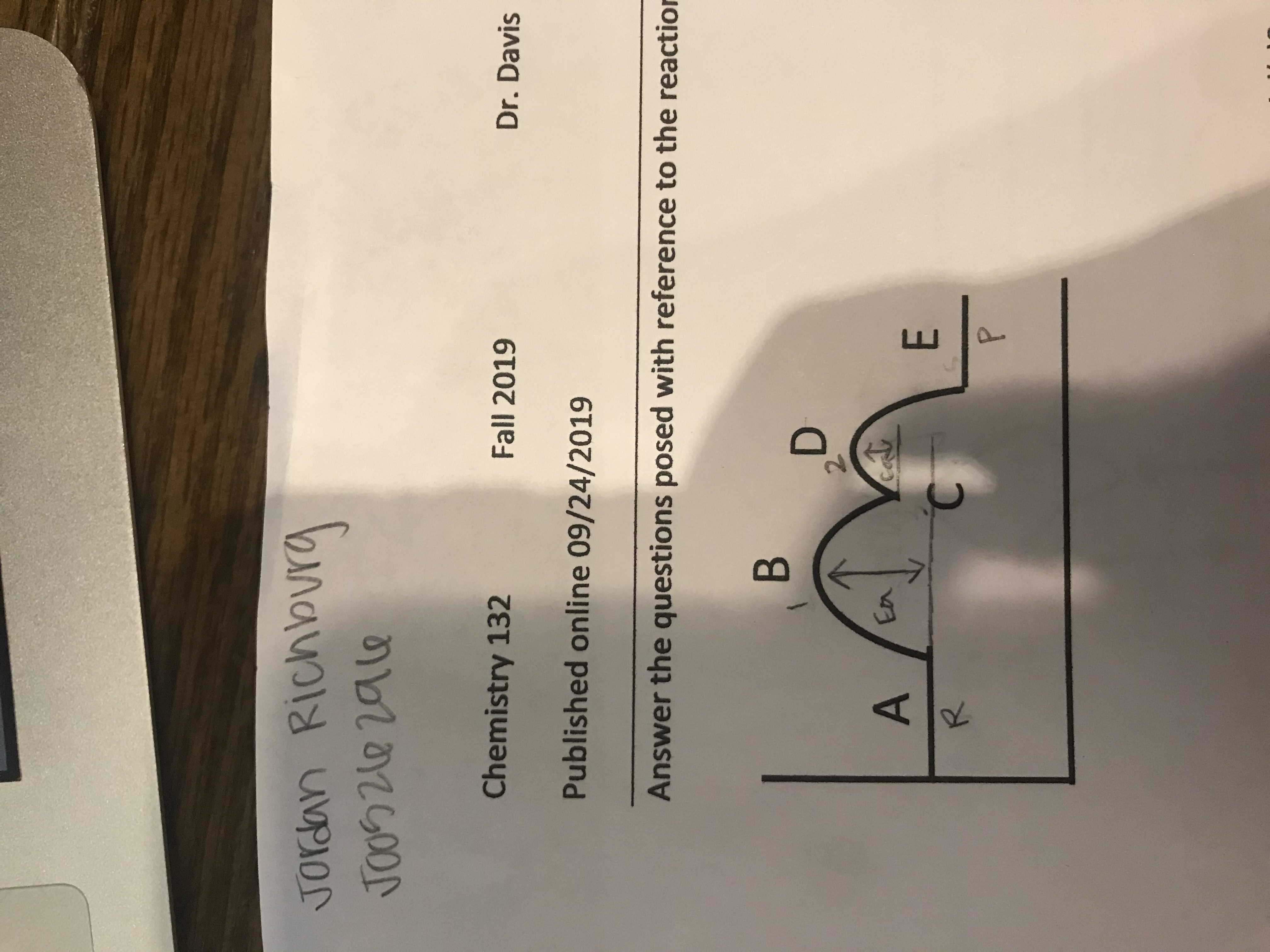
Write the equation for this reaction.
Write the general form of the rate law for this reaction.
which factor, if any, in the rate law changes if temperature is changed?
Transcribed Image Text:JOrdan Richlovra
Chemistry 132
Fall 2019
Dr.Davis
Published online 09/24/2019
Answer the questions posed with reference to the reaction
В
D
22
A
En
E
C
R
P
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution
Trending nowThis is a popular solution!
Step by stepSolved in 3 steps with 3 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- An elementary step is defined as a chemical collision in a reaction mechanism. A collection of different types of collisions makes up the reaction mechanism, so elementary steps provide a molecular view of the overall reaction. Write the rate law for the following reaction, which represents an elementary step in a reaction. Your rate law should not include the states of matter. $$SO2Cl2(g)SO2(g)+Cl2(g)arrow_forwardSome measurements of the initial rate of a certain reaction are given in the table below. Use this information to write a rate law for this reaction, and calculate the value of the rate constant k. Round your value for the rate constant to 3 significant digits. Also be sure your answer has the correct unit symbol.arrow_forwardSuppose the decomposition of ozone proceeds by the following mechanism: step elementary reaction rate constant 1 03(g) O₂(g) + 0(g) k₁ 2 03(g) + O(g) → 20₂(g) k₂ Suppose also k₁ »k₂. That is, the first step is much faster than the second. Write the balanced chemical equation for the overall chemical reaction: Write the experimentally- observable rate law for the overall chemical reaction. rate = k Note: your answer should not contain the concentrations of any intermediates. Express the rate constant k for the overall chemical reaction in terms of K₁, K2, and (if necessary) the rate constants k.1 and k-2 for the reverse of the two elementary reactions in the mechanism. = 0 k = 00 X 5 S ?arrow_forward
- Some measurements of the initial rate of a certain reaction are given in the table below. [N] 0.815M1.56M N,|H||initial rate of reaction 185. M/s 141.M/s Use this information to write a rate law for this reaction, and calculate the value of the rate constant k. Round your value for the rate constant to 3 significant digits. Also be sure your answer has the correct unit symbol. 口 rate = 0 ロ || = 1 Explanation Check D2022 McGrny Hl LLC. Al Rights Reserved. Terms of N 回 至 五arrow_forwardO KINETICS AND EQUILIBRIUM Deducing a rate law from initial reaction rate data Some measurements of the initial rate of a certain reaction are given in the table below. H, I, initial rate of reaction 0.735 M 0.260 M 78.0M/s 0.735 M 0.341 M 102. M/s 0.354 M 0.260 M 37.6M/s Use this information to write a rate law for this reaction, and calculate the value of the rate constant k. Round your value for the rate constant to 3 significant digits. Also be sure your answer has the correct unit symbol. Do rate = k| x10 k = Explanation Check © 2021 McGraw-Hill Educat IIarrow_forwardWhich of the following is not an example of heterogeneous catalysis? a) 2O3(g) → 3O2(g) has a catalyst of Cl(g) b) CO(g) + 3H2(g) → CH4(g)+H2O(g) has a catalyst of Co(s) c) C2H4(g) + H2(g) → C2H6(g) has a catalyst of Ni(s) d) 2CO(g) + 2NO(g) → 2CO2(g)+N2(g) has a catalyst Rh(s)arrow_forward
- = Using a rate law The rate of a certain reaction is given by the following rate law: rate = k[H₂ ] [NH₂] Use this information to answer the questions below. What is the reaction order in H₂? What is the reaction order in NH3? What is overall reaction order? At a certain concentration of H₂ and NH3, the initial rate of reaction is 17.0 M/s. What would the initial rate of the reaction be if the concentration of H₂ were doubled? Be sure your answer has the correct number of significant digits. The rate of the reaction is measured to be 2.0 x 10³ M/s when [H₂] = 0.75 M and [NH₂] = 0.44 M. Calculate the value of the rate constant. Be sure your answer has the correct number of significant digits. k= 0 0 04/ -1 X 1/5arrow_forwardThe rate of a certain reaction is given by the following rate law: rate = k[N,][H] Use this information to answer the questions below.arrow_forwardDeducing a rate law from initial reaction rate data Some measurements of the initial rate of a certain reaction are given in the table below. [N] [H] initial rate of reaction 0.977 M 1.26 M 0.162 M/s 2.87 M 1.26 M 1.40 M/s 0.977 M 2.13 M 0.274 M/s Use this information to write a rate law for this reaction, and calculate the value of the rate constant k. Round your value for the rate constant to 3 significant digits. Also be sure your answer has the correct unit symbol. rate = kO k = 0 Explanation Check O 2021 McGraw-Hill Education, All Rights Type here to search 立arrow_forward
- True or False _____ The kinetics of reaction deals with the energies of the products and the reactants. _____ Thermodynamics deals with reaction rates. _____ Understanding the reactions kinetics can help you understand the mechanism of a reaction. _____ Transition states are stable. _____ This is an example of an overall second order reaction (rate = kr[CH3Br][CH3-]) _____ The rate determining step is the step with the lowest activation energy.arrow_forwardDeducing a rate law from initial reaction rate data Some measurements of the initial rate of a certain reaction are given in the table below. N2 Hinitial rate of reaction 2.21 M 2.33 M 35.0 M/s 2.21 M 4.98 M 160. M/s 7.27 M 2.33 M 379. M/s Use this information to write a rate law for this reaction, and calculate the value of the rate constant k. Round your value for the rate constant to 3 significant digits. Also be sure your answer has the correct unit symbol. rate = k M k = Explanation Check 2021 McGraw Hill Educabon. All R P Type here to search 近arrow_forward
arrow_back_ios
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY