
Chemistry
10th Edition
ISBN: 9781305957404
Author: Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Question
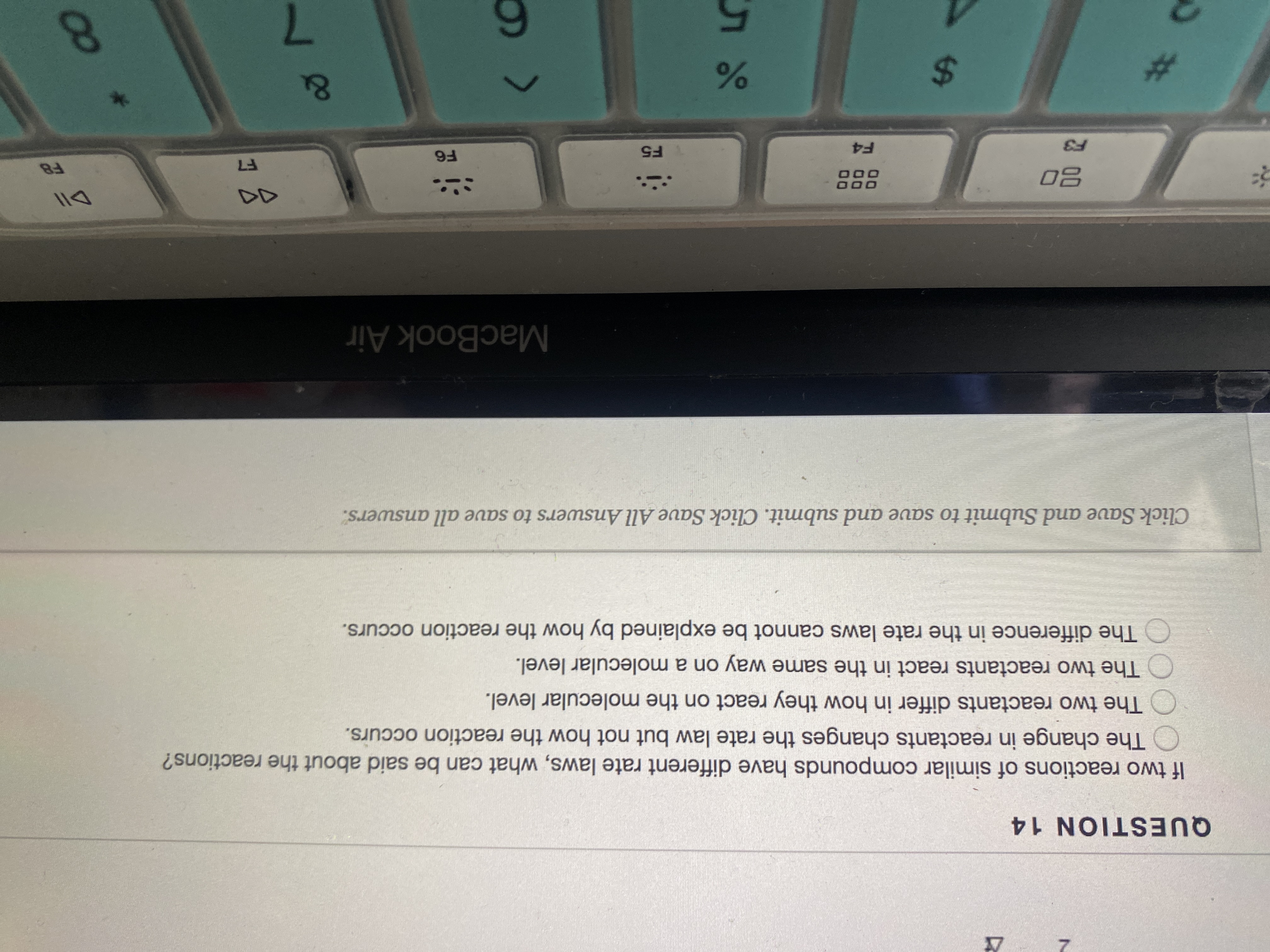
Transcribed Image Text:**Question 14:**
If two reactions of similar compounds have different rate laws, what can be said about the reactions?
- ○ The change in reactants changes the rate law but not how the reaction occurs.
- ○ The two reactants differ in how they react on the molecular level.
- ○ The two reactants react in the same way on a molecular level.
- ○ The difference in the rate laws cannot be explained by how the reaction occurs.
*Instructions: Click Save and Submit to save and submit. Click Save All Answers to save all answers.*
Expert Solution
arrow_forward
Step 1
Characteristics of rate law of a reaction
Step by stepSolved in 2 steps

Knowledge Booster
Similar questions
- Consider the decomposition of hydrogen iodide: HI-2 H₂+2 12 At 25 °C, the instantaneous rate of this reaction follows the rate law: Rate= (0.0016 M¹s ¹)[HI]² Suppose a vessel initially contains 1.15 MHI. What will be the [HI] concentration after the first 197.3 seconds of reaction. Report your answer to two significant figures without the unit. Example: 0.010 Type your answer..... Previousarrow_forwardA chemistry graduate student is studying the rate of this reaction: He fills a reaction vessel with and measures its concentration as the reaction proceeds: time (seconds) Use this data to answer the following questions. Write the rate law for this reaction. rate Calculate the value of the rate constant . Round your answer to significant digits. Also be sure your answer has the correct unit symbol.arrow_forward5arrow_forward
- Consider this reaction: 2N₂O5 (g) → 2N₂O4 (g) + O₂(g) At a certain temperature it obeys this rate law. = (0.291 M¹s¹) [N₂0₂] rate Suppose a vessel contains N₂O, at a concentration of 0.230 M. Calculate how long it takes for the concentration of N₂O to decrease to 23.0% of its initial value. You may assume no other reaction is important. Round your answer to 2 significant digits. ? Subm Continue Ⓒ2022 McGraw Hill LLC. All Rights Reserved. Terms of Use Privacy Cente 80°F Partly sunny ^ pe here to search B X Garrow_forwardConsider this reaction: 2N₂O5 (g) → 2N₂O4 (g) + O₂(g) At a certain temperature it obeys this rate law. rate = (0.0317 M¹-s¯¹) [₂0₂] S Suppose a vessel contains N₂O5 at a concentration of 0.440 M. Calculate the concentration of N₂O5 in the vessel 480. seconds later. You may assume no other reaction is important. Round your answer to 2 significant digits. M x10 ? X Sarrow_forwardFor the chemical reaction NO2(g) + CO(g) → NO(g) + CO2(g) The following initial rate data were obtained Initial [NO₂] Initial [CO] Initial Rate Experiment (mol/L) (mol/L) (mol/L.s) 1 0.10 0.10 0.0050 2 0.40 0.10 0.080 3 0.10 0.20 0.0050 What is the rate law for the reaction? R = k [NO₂]4[CO] R = k [NO₂][CO] R = k [NO₂]²[CO] none of the answers are correct OR = K [NO₂]²arrow_forward
- Consider this reaction: 2N₂O, (e)- 2N₂O₂(g) + O₂(g) At a certain temperature it obeys this rate law. rate - (0.0664 M¹¹)[N₂05] Suppose a vessel contains N₂O, at a concentration of 0.870M. Calculate how long it takes for the concentration of N₂O, to decrease to 16.0% of its initial value. You may assume no other reaction is important. Round your answer to 2 significant digits. 0.Parrow_forward2. Consider the reaction that occurs when a CIO2 solution and a solution containing hydroxide ions (OH) are mixed at 0°C, shown in the following equation. 2CIO2(aq) + 2OH (aq) → Cl03° (aq) + CIO2° (aq) + H2O (1) When solutions containing CIO2 and OH- in various concentrations were mixed at 0 oC, the following rate data were obtained: Determination Initial concentration Initial concentration of Initial rate for formation number of ClO2, mol/L OH", mol/L of CIO3¯ mol/Ls 1.25x10-2 1.30x10-3 2.33x10-4 2.50x10-2 1.30x10-3 9.34x104 3 2.50x10-2 2.60x103 1.87x10-3 a) Use the method of initial rates to find the order of the reaction with respect to CIO2 and with respect to OH. Write the rate equation for the reaction of CIO2 and OH´ at 0°C. b) Calculate the rate constant, k, for the reaction of clO2 and OH at 0°C. c) Calculate the reaction rate for the reaction CIO2 and OH at 0°C when the initial ClO2 and OH concentrations are 8.25x10-3 mol/L and 5.35x 10-² mol/L, respectively. 1.arrow_forward8. Smelling salt decomposes spontaneously at room temperature by the equation below. A student places a 10.00-g sample into a 1-liter sealed rigid vessel. What measurements should the student make to determine the rate of this reaction?(NH4)2CO3(s)→2NH3(g)+H2O(g)+CO2(g) a. change in mass and temperature b. temperature and time c. change in mass and time d. change in pressure and timearrow_forward
- I need help with question 10?arrow_forwardConsider this reaction: 2N₂O5 (g) → 2N₂O4 (g) +O₂ (g) At a certain temperature it obeys this rate law. rate = (0.0328 s−¹)[N₂05] S Suppose a vessel contains N₂O5 at a concentration of 0.860M. Calculate the concentration of N₂O5 in the vessel 22.0 seconds later. You may assume no other reaction is important. Round your answer to 2 significant digits. M x10 X Śarrow_forwardConsider this reaction: 2C1₂05 (g) → 2Cl₂ (g) +50₂ (g) At a certain temperature it obeys this rate law. rate =(0.0913 s¯¹)[C₁₂05] Suppose a vessel contains Cl₂05 at a concentration of 0.460M. Calculate the concentration of C1₂O5 in the vessel 9.70 seconds later. You may assume no other reaction is important. 25 2 Round your answer to 2 significant digits. [ M x10arrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY