
Chemistry
10th Edition
ISBN: 9781305957404
Author: Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Concept explainers
Question
thumb_up100%
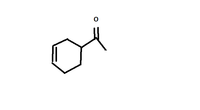
Is this compound sensitive to base or to acid? Explain.
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by stepSolved in 2 steps with 2 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- Arrange the numbered protons in decreasing order of acidity, explain the reasoning based upon the conjugate bases.arrow_forwardWhen this molecule is treated with a strong base, from which of the indicated sites is the hydrogen removed first? a b Select an answer and submit. For keyboard navigation, use the up/down arrow keys to select an answer. с A B C A B They are equally acidic. ZI NH Carrow_forwardProvide and label the conjugate base and acid. Is this reaction of product favored? Explain. (pKa H=35, H2O=15.7)arrow_forward
- Describe the acidity/basicity of each species and estimate the position of each equilibrium. CH3CH₂OH₂ CH3CH₂OH + a NH4 b and b is the On the left, a is the On the right, c is the and d is the The species favored at equilibrium are those C ◊ ◊ C + NH3 darrow_forwardList the given compounds in order of increasing acidity (lowest first). Give a reason for your answer.arrow_forwardwhich has the lowest ph at equilibrium? cyanic acid, formic acid, lactic acid, propionic acid, or benzoic acid?arrow_forward
- I need help with this problem. Compare the pH of propionic acid and nitric acid when they are dissolved in water at a concentration of 20 mM using the "Arrhenius Concept". Include:a balanced equation describing the acid-base reactions involvedThe calculation of the pKa for bothThe calculation of the two pH's (of propionic acid and nitric acid when they are dissolved in water at a concentration of 20 mM)and A calculation of the percent of dissociation of both propionic acid and nitric acid.The gibbs free energy ΔG are listed as the following:Nitric acid: -152 kJ/molPropionic Acid : -291 kJ/molPropionate: -361 kJ/molarrow_forward5.arrow_forwardDraw the conjugate acid (including any necessary charges) if the following compound reacts as a base.arrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY