
Chemistry
10th Edition
ISBN: 9781305957404
Author: Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Concept explainers
Question
thumb_up100%
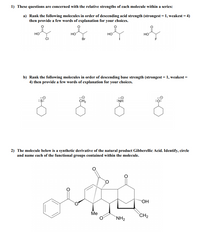
Transcribed Image Text:1) These questions are concerned with the relative strengths of each molecule within a series:
a) Rank the following molecules in order of descending acid strength (strongest = 1, weakest = 4)
then provide a few words of explanation for your choices.
HO
но
но
но
Br
b) Rank the following molecules in order of descending base strength (strongest = 1, weakest =
4) then provide a few words of explanation for your choices.
CH2
:NH
2) The molecule below is a synthetic derivative of the natural product Gibberellic Acid. Identify, circle
and name each of the functional groups contained within the molecule.
"OH
Me
CH2
`NH2
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution
Trending nowThis is a popular solution!
Step by stepSolved in 2 steps with 1 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- Complete the following table, which lists information about the measured acid dissociation constants of three unknown weak acids. Note: be sure each number you put in the table has the correct number of significant digits. K. pK. relative strength acid Dx10 v (Choose one) 1 (strongest) A 5.8 × 10 2 В 10.6 3 (weakest) C 8.258 (Choose one) ♥ B.arrow_forwardUse the table below to order the following from the strongest to the weakest acid. Formula Value of Ka HF 7.2 × 10-4 HOCI -8 HOCI 3.5 × 107 From the strongest to the weakest acid Drag and drop your selection from the following list to complete the answer: H₂O HC1 HFarrow_forwardQuestion 13 of 34 > Write the chemical equation for the reaction of carbonic acid (H₂CO3) with water. chemical equation:arrow_forward
- Complete the following table, which lists information about the measured acid dissociation constants of three unknown weak acids. Note: be sure each number you put in the table has the correct number of significant digits. acid Ka PK relative strength a x10 A ☐ 4.1 B ☐ 5.574 9 C 2.9 x 10 1 (strongest) 2 3 (weakest)arrow_forward14. Consider the Bronsted-Lowry acid/base reaction below. Ⓒ acide O-H Br a. b. C. + X Br Con acide Base Con Baçe Draw the products and label the acid, base, conjugate acid, conjugate base Use curved arrows to show the movement of electrons to form the products. (Draw in missing lone pair electrons, or hydrogens if they are involved) Does equilibrium favor the starting material or products? Why? Explain your answer with structures and words. it would farver the starting as it's more stable X Whyarrow_forwardWhat is the order of acid strength of the following molecules? 1 CH,COమH 2 CH3CH,COH 3 CH,BRCO,H 4 CH,FCO,H A) 2>1>4>3 B) 4>3>1>2 C) 3-4>2>1 D) 4>3>2>1arrow_forward
- larrow_forwardComplete the following table, which lists information about the measured acid dissociation constants of three unknown weak acids. Note: be sure each number you put in the table has the correct number of significant digits. acid A B C Ka 4.29 X 10 1.58 × 10 -7 - 11 9.12 × 10 -3 pKa 6.37 10.8 2.04 relative strength 2 3 (weakest) 1 (strong ↑ x10 Be Careful Please check the number of significant digits in your answer. OKarrow_forwardComplete the following table, which lists information about the measured acid dissociation constants of three unknown weak acids. Note: be sure each number you put in the table has the correct number of significant digits. acid A B ( C Ka 0 3.3 X 10 -4 pKa 1.789 6.2 relative strength (Choose one) (Choose one) 9 (Choose one) B Xarrow_forward
- Complete the following table, which lists information about the measured acid dissociation constants of three unknown weak acids. Note: be sure each number you put in the table has the correct number of significant digits. acid A B C Ka 2. X 10 - 12 3.94 X 10 - 10 pK a 9.02 relative strength 1 (strongest) 2 3 (weakest) x10 X Śarrow_forwardDo not give handwriting solution.arrow_forwardArrange the following in terms of increasing stability in section IV, acidity in section V, and basicity in section VI using the less than (<) inequality.arrow_forward
arrow_back_ios
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY