
Introduction to Chemical Engineering Thermodynamics
8th Edition
ISBN: 9781259696527
Author: J.M. Smith Termodinamica en ingenieria quimica, Hendrick C Van Ness, Michael Abbott, Mark Swihart
Publisher: McGraw-Hill Education
expand_more
expand_more
format_list_bulleted
Question
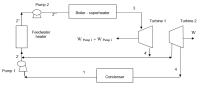
In the Rankine cycle with a feedwater heater as shown in the figure, the condensing temperature T1 is 25oC. The superheat temperature T3 is 370oC and the vaporization pressure P3 is 32 bar. Enough steam is bled from Turbine 1 at 7 bar (P2’) to heat the feedwater 2’ to a saturated liquid 2’’. Determine the thermal efficiency of this reversible cycle and compare it to a Carnot cycle operating between the same two temperature extremes. Show the cycle in a T-s diagram. Also compute the net work output per kilogram of steam.
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution
Trending nowThis is a popular solution!
Step by stepSolved in 10 steps with 20 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemical-engineering and related others by exploring similar questions and additional content below.Similar questions
- 2. Double pipe heat exchanger is used to heat water from 20 ° C to 80 ° C the flow rate is 1.2 kg / s. The hot fluid is hot water entering the double pipe at 160 ° C at a flow rate of 2 kg / s. Overall heat transfer coefficient 640 W / m². K. Cp of hot water = 4.31 kJ / kg. K and Cp of cold water = 4.18 kJ / kg. K a. Calculate the maximum heat transferred in this system b. What is the actual heat transferred c. What is the effectiveness of this tool d. Calculate NTU e. What is the surface area of the heat transferarrow_forwardWater vapor expands at a turbine at a speed of 1350 kg / s, the inlet conditions are 8MPA and 400C, the outlet conditions are 500KPa and 250C, the turbine does 200kW work. Neglect changes in kinetic and potential energy. a) Write and simplify the equation that applies to this system. b) What are the specific enthalpies of the input and output current? c) What is the heat transfer in Kw? d) What is the specific volume and volumetric flow at the inlet? e) What is the specific volume and the volumetric flow at the outletarrow_forwardAir is contained in a piston cylinder arrangement as undergoes a cycle as shown in the figure. The initial temperature T1 = 800oF and the amount of air is 1 lbm. The process from 3 to 1 is adiabatic. Determine the total work and the net heat transfer in Btu. Assume constant heat capacities.arrow_forward
- Question 1.2 A helical coil cooler is used to cool 0.5 kg.s¹ of hot oil from 100°C down to 40°C. Cooling water with an inlet temperature of 10°C flows inside the coiled tube. The specific heat of the oil is 2 kJ.kg¹.K¹, the specific heat of the water is 4.2 kJ.kg¹.K¹, and the density of water is 1000 kg.m. a) Determine the lowest possible cooling water flow rate. (0.48 kg.s¹) b) Determine the inside diameter of the tube if a cooling water flow of 1.5 times the minimum is used, and the recommended flow velocity is 1 m.s. Select an appropriate tube from the following standard sizes: Outside diameter (mm) Wall thickness (mm) 30 1.5 35 2.0 40 2.0 45 2.5 50 3.0 c) For a heat transfer coefficient of U = 800 W.m2.K, determine the heat transfer surface area and the tube length of the heat exchanger. The oil inside the tank is mixed by a stirrer and the oil temperature at any location in the tank can be assumed to be equal to the oil outlet temperature. (Area = 4.12 m², length = 39.7 m)arrow_forwardA compressor is to compress 5.0 mol/s of air at 20°C and 200 kPa to 800 kPa. The ratio ofconstant pressure to constant volume heat capacity for air is 1.4. Determine the following:a) Power (in kW) required if the mechanical efficiency of the uncooled compressor is 70%.b) Outlet temperature of the uncooled compressor and whether it is safe for operation.c) The percentage of power saved if the compressor were run isothermally.arrow_forwardA flow of 1950 kg water/h enters at 290 K for cooling and flow outside the tube. An oil which has a heat capacity is 2.30 kJ/(kg∙K) is being cooled in a heat exchanger from 372 K to 310 K and flows inside the tube at a rate of 2600 kg/h. Calculate the water outlet temperature and heat transfer area if the overall heat transfer coefficient is 340 W/(m2·K) and the streams are countercurrent.arrow_forward
arrow_back_ios
arrow_forward_ios
Recommended textbooks for you
 Introduction to Chemical Engineering Thermodynami...Chemical EngineeringISBN:9781259696527Author:J.M. Smith Termodinamica en ingenieria quimica, Hendrick C Van Ness, Michael Abbott, Mark SwihartPublisher:McGraw-Hill Education
Introduction to Chemical Engineering Thermodynami...Chemical EngineeringISBN:9781259696527Author:J.M. Smith Termodinamica en ingenieria quimica, Hendrick C Van Ness, Michael Abbott, Mark SwihartPublisher:McGraw-Hill Education Elementary Principles of Chemical Processes, Bind...Chemical EngineeringISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...Chemical EngineeringISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY Elements of Chemical Reaction Engineering (5th Ed...Chemical EngineeringISBN:9780133887518Author:H. Scott FoglerPublisher:Prentice Hall
Elements of Chemical Reaction Engineering (5th Ed...Chemical EngineeringISBN:9780133887518Author:H. Scott FoglerPublisher:Prentice Hall
 Industrial Plastics: Theory and ApplicationsChemical EngineeringISBN:9781285061238Author:Lokensgard, ErikPublisher:Delmar Cengage Learning
Industrial Plastics: Theory and ApplicationsChemical EngineeringISBN:9781285061238Author:Lokensgard, ErikPublisher:Delmar Cengage Learning Unit Operations of Chemical EngineeringChemical EngineeringISBN:9780072848236Author:Warren McCabe, Julian C. Smith, Peter HarriottPublisher:McGraw-Hill Companies, The
Unit Operations of Chemical EngineeringChemical EngineeringISBN:9780072848236Author:Warren McCabe, Julian C. Smith, Peter HarriottPublisher:McGraw-Hill Companies, The

Introduction to Chemical Engineering Thermodynami...
Chemical Engineering
ISBN:9781259696527
Author:J.M. Smith Termodinamica en ingenieria quimica, Hendrick C Van Ness, Michael Abbott, Mark Swihart
Publisher:McGraw-Hill Education

Elementary Principles of Chemical Processes, Bind...
Chemical Engineering
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY

Elements of Chemical Reaction Engineering (5th Ed...
Chemical Engineering
ISBN:9780133887518
Author:H. Scott Fogler
Publisher:Prentice Hall


Industrial Plastics: Theory and Applications
Chemical Engineering
ISBN:9781285061238
Author:Lokensgard, Erik
Publisher:Delmar Cengage Learning

Unit Operations of Chemical Engineering
Chemical Engineering
ISBN:9780072848236
Author:Warren McCabe, Julian C. Smith, Peter Harriott
Publisher:McGraw-Hill Companies, The