
Introduction to Chemical Engineering Thermodynamics
8th Edition
ISBN: 9781259696527
Author: J.M. Smith Termodinamica en ingenieria quimica, Hendrick C Van Ness, Michael Abbott, Mark Swihart
Publisher: McGraw-Hill Education
expand_more
expand_more
format_list_bulleted
Question
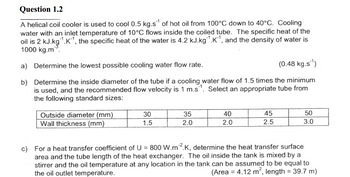
Transcribed Image Text:Question 1.2
A helical coil cooler is used to cool 0.5 kg.s¹ of hot oil from 100°C down to 40°C. Cooling
water with an inlet temperature of 10°C flows inside the coiled tube. The specific heat of the
oil is 2 kJ.kg¹.K¹, the specific heat of the water is 4.2 kJ.kg¹.K¹, and the density of water is
1000 kg.m.
a) Determine the lowest possible cooling water flow rate.
(0.48 kg.s¹)
b)
Determine the inside diameter of the tube if a cooling water flow of 1.5 times the minimum
is used, and the recommended flow velocity is 1 m.s. Select an appropriate tube from
the following standard sizes:
Outside diameter (mm)
Wall thickness (mm)
30
1.5
35
2.0
40
2.0
45
2.5
50
3.0
c) For a heat transfer coefficient of U = 800 W.m2.K, determine the heat transfer surface
area and the tube length of the heat exchanger. The oil inside the tank is mixed by a
stirrer and the oil temperature at any location in the tank can be assumed to be equal to
the oil outlet temperature.
(Area = 4.12 m², length = 39.7 m)
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by stepSolved in 4 steps

Knowledge Booster
Similar questions
- 1. Assume steady-state conditions and a thermal conductivity of k 1.5 W/m-K. a. Determine the temperatures at nodes 1, 2, and 3 b. Determine the heat transfer rate/(unit thickness into the page) from the system to the convective fluid. Insulated boundary 129.4 T2 45.8 T = 30°C h = 50 W/m2-K 0.1 m T3 137.0 103.5 0.1 m 172.9 T1 132.8 67,0 Isothermal boundary To = 200°Carrow_forwardA 5 cm thick steel pipe, 1.0 m long, with an internal diameter of 8 cm is covered with 4 cm thick insulation. The inside wall temperature of the steel pipe is 100°C. The ambient temperature around the insulated pipe is 24°C. The convective heat-transfer coeffi cient on the outer insulated surface is 50 W/(m² K). Calculate the temperature at the steel insulation interface. The thermal conductivity of steel is 54 W/(m K), and the thermal conductivity of insulation is 0.04 W/(m K).arrow_forwardLiquid food is heated in a tubular heat exchanger. The inner pipe wall temperature is 110 ° C. The internal diameter of the pipe is 40 mm. Product flows at 0.7 kg / s. If the initial temperature of the product is 7 ° C, calculate the convective heat transfer coefficient. The thermal properties of the product are as follows: specific heat = 3.7 kJ / (kg ° C), thermal conductivity = 0.6 W / (m ° C), product viscosity = 500 x 10-6 Pa s, density = 1000 kg / m³ , the product viscosity at 110 ° C = 410 x 10-6 Pa s. a. Find the Reynold number = Answer. b. Find the number Prantl = Answer. c. Find the Nuselt = Answer number d. Convection coefficient = AnswerW / m² ° C.arrow_forward
- A rubber insulated copper wire 2 mm diameter is carrying electric current and exposed to a uniform temperature of air at 40°C. What will be the maximum steady current in amperes that can be allowed if the rubber insulation is not to get heated above 45°C? Insulation thickness=1 mm Thermal conductivity of rubber= 0.15 W/m-K Air film coefficient= 10 W/m2-K Electrical resistivity of copper=10-7 ohm-m A 10.45 A В 2.1 A C) 14.5 A D 4.25 A E 9.65 A F None of thesearrow_forward4arrow_forward5. A laboratory water bath has an immersed horizontal (cylindrical) water element, 2.54 cm. in diameter and 35cm in length, with a power input of 500 W. If the bulk water temperature is 40 °C and heat transfer occurs by free convection only, calculate the surface temperature of the heater.arrow_forward
- Hollow Sphere Saturated steam at 267°F is flowing inside a hollow sphere with an ID of 0.824 in. and an OD of 1.05 in. The pipe is insulated with 1.5 in. of insulation on the outside. The convective heat transfer coefficient inside and outside the pipe is hi = 1000 Btu/hr/ft2/°F and ho = 2 Btu/hr/ft2/°F, respectively. The mean thermal conductivity of the metal is 45 W/m/K or 26 Btu/hr/ft/°F, while that of the insulation material is 0.064 W/m/K or 0.037 Btu/hr/ft/°F. a. Calculate the heat loss for 1 ft of pipe using resistances if the surrounding air is at 80°F. b. Calculate the temperature profile developed for this system. c. Calculate the inside and outside overall heat transfer coefficients.arrow_forwardProblem 3 A novel material is suddenly exposed on one side to a fluid material. The material has an overall thermal gradient of °C W -7,000 with a thermal conductivity of 23.8. The fluid m m.K • has a temperature of 30° C and a heat transfer coefficient of 325 W m². K. material. . Calculate the temperature at the surface of thearrow_forwardÀ wall of area 30 m² having a density of 1500 kg/m', thermal conductivity 30 W/m.K, and specific heat capacity 4 kJ/kg.K. The temperature distribution across a wall 0.5 m thick at a certain instant of time is given as T(x) = 30-5 x-7x The wall is generating a uniform heat (q.) of 1000 W. (1) Find the rate of heat transfer entering and leaving the wall (in W). (2) Find rate of energy stored in Watt. (3) Find (dFT/dx²) (4) Derive the change in temperature with respect to time equation (time rate of temperature change)- remember to substitute the value of (d T/dx²) from (part 3) and values of all other properties into final equation. %3Darrow_forward
- Steel pipe (outer diameter 100 mm) is covered with two layers of insulation. The inner layer, 40 mm thick, has a thermal conductivity of 0.07 W / (m K). The outer layer, 20 mm thick, has a thermal conductivity of 0.15 W / (m K). Pipes are used to deliver steam with a pressure of 700 kPa. The temperature on the outer insulation surface is 24 ° C. If the pipe is 10 m long, determine the following: (assuming that the conduction heat transfer resistance of the steel pipe and the vapor convection resistance are negligible). a. Heat loss per hour. = AnswerkJ / hour. b. Temperature between insulation layers. = Answer° C.arrow_forwardneed the correct ansarrow_forwardPlease answer Aarrow_forward
arrow_back_ios
arrow_forward_ios
Recommended textbooks for you
 Introduction to Chemical Engineering Thermodynami...Chemical EngineeringISBN:9781259696527Author:J.M. Smith Termodinamica en ingenieria quimica, Hendrick C Van Ness, Michael Abbott, Mark SwihartPublisher:McGraw-Hill Education
Introduction to Chemical Engineering Thermodynami...Chemical EngineeringISBN:9781259696527Author:J.M. Smith Termodinamica en ingenieria quimica, Hendrick C Van Ness, Michael Abbott, Mark SwihartPublisher:McGraw-Hill Education Elementary Principles of Chemical Processes, Bind...Chemical EngineeringISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...Chemical EngineeringISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY Elements of Chemical Reaction Engineering (5th Ed...Chemical EngineeringISBN:9780133887518Author:H. Scott FoglerPublisher:Prentice Hall
Elements of Chemical Reaction Engineering (5th Ed...Chemical EngineeringISBN:9780133887518Author:H. Scott FoglerPublisher:Prentice Hall
 Industrial Plastics: Theory and ApplicationsChemical EngineeringISBN:9781285061238Author:Lokensgard, ErikPublisher:Delmar Cengage Learning
Industrial Plastics: Theory and ApplicationsChemical EngineeringISBN:9781285061238Author:Lokensgard, ErikPublisher:Delmar Cengage Learning Unit Operations of Chemical EngineeringChemical EngineeringISBN:9780072848236Author:Warren McCabe, Julian C. Smith, Peter HarriottPublisher:McGraw-Hill Companies, The
Unit Operations of Chemical EngineeringChemical EngineeringISBN:9780072848236Author:Warren McCabe, Julian C. Smith, Peter HarriottPublisher:McGraw-Hill Companies, The

Introduction to Chemical Engineering Thermodynami...
Chemical Engineering
ISBN:9781259696527
Author:J.M. Smith Termodinamica en ingenieria quimica, Hendrick C Van Ness, Michael Abbott, Mark Swihart
Publisher:McGraw-Hill Education

Elementary Principles of Chemical Processes, Bind...
Chemical Engineering
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY

Elements of Chemical Reaction Engineering (5th Ed...
Chemical Engineering
ISBN:9780133887518
Author:H. Scott Fogler
Publisher:Prentice Hall


Industrial Plastics: Theory and Applications
Chemical Engineering
ISBN:9781285061238
Author:Lokensgard, Erik
Publisher:Delmar Cengage Learning

Unit Operations of Chemical Engineering
Chemical Engineering
ISBN:9780072848236
Author:Warren McCabe, Julian C. Smith, Peter Harriott
Publisher:McGraw-Hill Companies, The