
Chemistry
10th Edition
ISBN: 9781305957404
Author: Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Concept explainers
Question
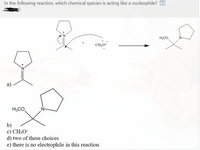
Transcribed Image Text:In the following reaction, which chemical species is acting like a nucleophile? 5
H3CO
CH;O
a)
H3CO
b)
c) CH3O¯
d) two of these choices
e) there is no electrophile in this reaction
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution
Trending nowThis is a popular solution!
Step by stepSolved in 3 steps with 1 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- What is the first step of the mechanism when a strong nucleophile adds to a ketone? a) Protonation of the C=O oxygen b) Deprotonation of the C-H hydrogen c) Nucleophilic addition to the C=O carbon d) Nucleophilic addition to the C=O oxygenarrow_forwardi) Starting compounds Br HC=CH ii) Br CH3 "OH Br iii) a. the only productarrow_forward6S. What is the major product of this reaction? 65. NaOH a C d.arrow_forward
- 2- Draw the mechanism of the following reaction (show the arrow pushing). Он 1. NaOH Me + H3C H. CH3 2. H30 Me A) Mechanism? B) What is the name of the reaction?arrow_forwardi H Br OCH 3 step 1 Br-Br step 2 Br B D A C In step 2 of the reaction above, classify C and D as electrophile or nucleophile. Both C and D are acting as electrophiles. Both C and D are acting as nucleophiles. C is acting as the nucleophile, D is acting as the electrophile. OC is acting as the electrophile, D is acting as the nucleophile. HOCH 3arrow_forward31aarrow_forward
- 51arrow_forwardH FCEC Organic product Inorganic product + + H Br Draw the organic product. Draw the inorganic product. Select Draw Rings More Erase Select Draw Rings More Erase H Br H Br Select the statement that properly identifies the nucleophile, substrate, and leaving group. O Br is the substrate, CH,C=C:- is the nucleophile, and CH, Br is the leaving group. O CH,C=C: is the substrate, CH, Br is the nucleophile, and Br is the leaving group. O CH, Br is the substrate, CH,C=C: is the nucleophile, and Br is the leaving group.arrow_forward4) What is the product of this reaction? A) ОН + B) OH Д ? он OH :0 c) род к D) OH 9000 7 8 OH 11arrow_forward
- Under the right conditions, hydrochloric acid, HCI, can react with alkenes. Which drawing shows the correct polarity of the H-CI bond and does HCI act as a nucleophile or electrophile? Select one: a. + H-CI Nucleophile Ob. This molecule does not have polar bonds, but acts as an electrophile. ○ C. + Electrophile CI- -H d. + Electrophile H-CI e. + Nucleophile CI-Harrow_forwardQuestion 22 Rank the following molecules in order of increasing relative rate of an SN1 reaction with methanol and heat (slowest to fastest reacting). ÇI Br Br Br CH3 ČH3 CH3 CH3 H;C Br 1 2 4 O 2<3<4<1<5 O1<2<5< 4 <3 O5<4<3< 2<1 O 2<3<4< 5 < 1 O 3<2<4< 5 < 1arrow_forwardHighlight the electrophilic carbon in red, and highlight the leaving group in blue. Highlight the atom in the nucleophile that will attack the electrophilic center in green. Only atoms need to be highlighted and not the lone pairs or formal charges. O O − Clarrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY