
Chemistry
10th Edition
ISBN: 9781305957404
Author: Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Concept explainers
Question
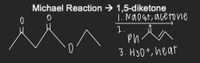
Transcribed Image Text:Michael Reaction → 1,5-diketone
1. Nao at, acetone
2.
3. H30+, heat
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by stepSolved in 2 steps with 2 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- What are these 2?arrow_forward108. For each set of structures, determine whether the two 112. How many kilograms of CO2 are produced by the com- 110. Complete the equation (assume only one addition). plete combustion of 3.8 kg of n-octane? Hint: Begin by writ- structures are isomers or the same molecule drawn in different ways. (a) || H;C-C-0-CH2–CH; H3C-CH,-C-0-CH3 (b) H3C-C=C-CH,-CH3 H3C-CH2-C=c-CH; (c) H3C-C-OH НО — С—СНз CH3 CH2 CH CH3 CH=CH + HI ms ne? dro- ing a balanced equation for tho of n-octane. O=Uarrow_forward(a) • All hydrogen atoms are implied. • You do not have to consider stereochemistry. • Omit lone pairs and radical electrons from your answer. 3-ethyl-3-pentanol Draw the structure of the alkene that was used to prepare the alcohol in highest yield. -8) O GA + H₂C * Cat CH₂ ? ChemDoodle Which process does this employ? 01. Hg(OAc)2, H₂O; 2. NaBH4 01. BH3; 2. H₂O2, NaOH OOSO4, H₂O2 Yarrow_forward
- N O CH3 Br 2-methylpropene/H 2SO4 (cat) heat AICI3 CI C14H19Brarrow_forward2-methylbutan-1-ol + [O] ----> ? (For tertiary alcohols ONLY draw reactant since there is no reaction)arrow_forwardWrite equations for the reaction of 2-butanol with reagent. Where you predict no reaction, write NR. Q)HBr, heatarrow_forward
arrow_back_ios
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY