
Chemistry
10th Edition
ISBN: 9781305957404
Author: Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Concept explainers
Question
31a
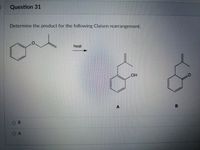
Transcribed Image Text:Question 31
Determine the product for the following Claisen rearrangement.
heat
OH
A
B.
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by stepSolved in 2 steps with 1 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- You were assigned to assay a product sample of milk of magnesia. A 0.600-g sample was reacted with 25.00 mL 0.10590 N H2SO4 . The excess unreacted acid in the solution required 13.00 mL of 0.09500 N NaOH when titrated to reach the methyl red end point. Determine the dosage strength of the product in terms of % Mg(OH)2 content. Type your answer in 2 decimal places, numbers only.arrow_forward24 25 26 A chemistry student weighs out 0.0202 g of acrylic acid (HCH,CHCO,) into a 250. mL volumetric flask and dilutes to the mark with distilled water. He plans to titrate the acid with 0.1800M NAOH solution. Calculate the volume of NaOH solution the student will need to add to reach the equivalence point. Round your answer to 3 significant digits. mL x10 Continue Submit Assignment 2021 McGraw Hill LLC. All Rights Reserved. Terms of Use Privacy Center | Accessibility C 80 000 000 F1 F2 DII DD F3 F4 F5 F6 F7 F8 F9 F10 F11 F12 @ 2$4 % &arrow_forwardAnalytical chemistryarrow_forward
- PLEASE PROVIDE DETAILED SOLUTION AND GIVE THE EXPLANATION OF CORRECT AND INCORRECT OPTIONS....arrow_forwardWhile you are working in a hospital laboratory, a patient complaining of severe stomach cramps and labored respiration dies within minutes of being admitted. Relatives of the patient tell you that he may have ingested rat poison. Therefore, you have his stomach pumped to verify this and to determine the cause of death. One of the more logical things to do would be to attempt to isolate the agent that caused death and perform chemical analyses on it. Assume that this was done, and the analyses showed that the isolated chemical compound contained, by weight, 60.0% potassium, 18.5% carbon, and 21.5% nitrogen. What is the chemical formula for this compound?arrow_forward22 23 24 25 26 21 16 17 18 19 20 15 A chemistry student weighs out 0.321 g of citric acid (H,C,H,0,), a triprotic acid, into a 250. mL volumetric flask and dilutes to the mark with distilled water. He plans to titrate the acid with 0.2000M NaOH solution. Calculate the volume of NaOH solution the student will need to add to reach the final equivalence point. Round your answer to 3 significant digits. Ar I mL x10 Submit Assignment Continue Terms of Use Privacy Center Accessibility 2022 McGraw Hill LLC. All Rights Reserved. Show A IMG-6095.jpg IMG-6096.jpg IMG-6097.jpg IMG-6098.jpg IMG-6099.jpg MacBook Air DII F12 F11 F9 F10 80 E7 F8 F6 F5 F4 esc F2 F3 F1 & @ # $ % 4 5 6 7 8 1 2 3 P Q W E R т tab * 21arrow_forward
- barkamishen seslb to doing Bittetsbau nA sideT oibore peivab ot etsimodo it as lendas suchombre or to agninnigod adr oldmseen polls anoitoleaimora ni baya Solovi immediabudinng enoitonen Isolmarlo lo coqyt Istoresbout wot ybuta lliw s Croborecho ofnino2s1 ayow ribi of 5) Analysis of 1.618 g of a hydrate salt gave the following information. Iliw Pb: 0.884 g, C: 0.205 g, H: 0.026 g, O: 0.273 g, and water: 0.230 g.o Inters What is the formula of the hydrate. sob a ogrobimu snocheoid bas in botser asce ed with asmolo s nad oson inmoasigaib to savi Inomalo srit 'il bogoo oinai is gaininos nouutos of babbe smogod bis noi si di autosis syneriozs Irw ticodulos ni noi ni nobnice to bilo 25ml produced flash and poppe carolionoriconic lieth noitonen inomossigen sigme yd elas Jabot ovi HA obrtw scale 6 10 enolonias pozemol adi ni ailuas, noitufos at devan gods got abou odregos bazum o obnoldo miuiboa bar otain rovlie to anoituloa asrl W SiT moironen insmoelor siduob a lo loss of an emot…arrow_forwardpls answer all of them.arrow_forward0 Cals) + NAC lag) – O White the balanced molecular chemical equation for the reachion f Solid lithium with aqueo us Chromium (111) acetat: 4 no iea chun occurs, write UR. Idaite a balanced molecular Chemical equahion for the Solia Sodium with aqueous Zinc perchlorite . 7 no geaction ccuns,arrow_forward
arrow_back_ios
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY