
Chemistry
10th Edition
ISBN: 9781305957404
Author: Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Question
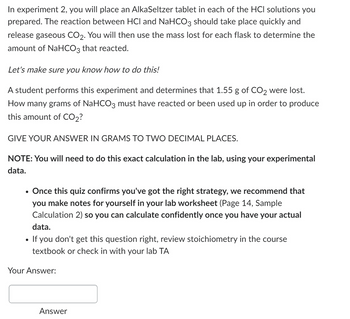
Transcribed Image Text:In experiment 2, you will place an AlkaSeltzer tablet in each of the HCI solutions you
prepared. The reaction between HCI and NaHCO3 should take place quickly and
release gaseous CO2. You will then use the mass lost for each flask to determine the
amount of NaHCO3 that reacted.
Let's make sure you know how to do this!
A student performs this experiment and determines that 1.55 g of CO2 were lost.
How many grams of NaHCO3 must have reacted or been used up in order to produce
this amount of CO2?
GIVE YOUR ANSWER IN GRAMS TO TWO DECIMAL PLACES.
NOTE: You will need to do this exact calculation in the lab, using your experimental
data.
•
•
Once this quiz confirms you've got the right strategy, we recommend that
you make notes for yourself in your lab worksheet (Page 14, Sample
Calculation 2) so you can calculate confidently once you have your actual
data.
If you don't get this question right, review stoichiometry in the course
textbook or check in with your lab TA
Your Answer:
Answer
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by stepSolved in 2 steps with 1 images

Knowledge Booster
Similar questions
- Classify each chemical reaction: Reaction Cl₂(g) + 2KI (aq) → 2KCl(aq) + 1₂ (s) H₂SO₂ (aq) H₂O(l) + SO₂ (g) PbCl₂ (aq) + FeSO (aq) → FeCl₂ (aq) + PbSO₂ (s) Mg(s) + F₂ (g) → MgF₂ (s) Type choose one choose one choose one choose one X Śarrow_forwardList the types of chemical reactions, include the generic equations (go back to the video in Module 9 about Types of Chemical Reactions if you need to refresh your memory, here is more practice, if you want to master the topic) . Classify the following chemical reactions in all possible ways (some of the chemical equations are not balanced, do NOT worry about it): C(s) + O₂(g) →→→ CO₂(g) Na(s) + Cl₂(g) →→→ NaCl(g) O H₂O(1) →H₂(g) + O2(8) CaCO3(s) CaO(s) + COz(3) CH4(8) + O₂(8) CO₂(g) + H₂O(g) O C3H8(g) + O2(8) CO2(g) + H₂O(g) O O O O C₂H5OH() + O2(8) Fe(s) + CuCl₂(aq) Cu(s) + AgNO3(aq) BaClz(aq) + Na,SO4(aq) O KBr(aq) +AgNO3(aq) O - O - CO2(8)+ H₂O(g) Cu(s) + FeCl₂(aq) Ag(s) + Cu(NO3)2(aq) →BaSO4(s)+ NaCl(aq) AgBr(s) + KNO3(aa)arrow_forwardClassify each chemical reaction: Reaction Ba (C1O3)₂ (s) → BaCl₂ (s) + 30₂ (g) 2 2Crl₂(aq) + 3Pb (NO3)₂ (aq) → 2Cr(NO3), (aq) + 3PbI₂ (s) 2 2Al(s) + 3ZnBr₂ (aq) 2A1Br, (aq) + 3Zn(s) Type choose one choose one choose one ↑ ŵ ↑arrow_forward
- Consider the general chemical equation 3A+B→2C. Part A. If 1.45 g of A reacts with 1.75 g of B, what is the mass of C? Express your answer with the appropriate units. Part B. If 4.70 g of A reacts to produce 8.95 g of C, what is the mass of B? Express your answer with the appropriate units.arrow_forwardFrom the list of reactions below, pick out the combustion reactions? 1) 2C2H6(g) + 7O2 (g) → 4CO2(g) + 6H2Ol) 2) MgO(s) + CO2 (g) → MgCO3(s) 3) ZnCO3(s) → ZnO (s) + CO2 (g) 4) 2CH3OH(l) + 3O2 (g) → 2CO2(g) + 4H2O(l) a 1, 2, 3, and 4 b 3 and 4 c 2, 3, and 4 d 1, 3, and 4 e 1 and 4arrow_forward13. Aluminum metal (Al) reacts with sulfur (S) to produce aluminum sulfide (ALS) according to this balanced chemical equation: 2 Al(s) + 3 S(s) → AlS(s) How many atoms of aluminum will react completely with 1.33 x 10 atoms of sulfur?arrow_forward
- Struggling to rewrite the equationarrow_forwardBalance the following equations (be sure to first convert any chemical names into formulas). Show all steps for balancing or write a brief explanation of your mental process if you were able to balance it in your head. FeCl3 + NaOH → Fe(OH)3 + NaCl P + O2 → P2O5 Lead(II) hydroxide reacts with hydrogen chloride to produce water and lead(II) chloride. Zinc sulfide reacts with aluminum phosphide to produce zinc phosphide and aluminum sulfide.arrow_forwardBalance the following chemical reaction. Enter the sum of the balanced coefficients as your answer. Assign "blank" coefficients a value of 1. ammonia + oxygen gas -→ nitrogen monoride + waterarrow_forward
- Here is some information to help. In this experiment, magnesium metal is heated until it reacts with oxygen in the air according to the following balanced equation: 2Mg(s)+ O2(g) → 2MgO(s) (silver metal) (white-gray ash) As the magnesium burns in air, it may also combine with nitrogen in air. To remove any nitride product, water is added, and the product is reheated. Any nitride product is converted to magnesium oxide and ammonia. 3Mg(s)+ N2(g) → Mg3N2(s) Mg3N2(s)+3H2O(l)→3MgO(s)+2NH3(aq) A weighed amount of the magnesium metal is used, and the mass of the oxide product formed is determined at the end of the experiment. The mass of oxygen that combines with the magnesium is obtained from the difference between the mass of the oxide product and the original mass of magnesium. mass of oxide product-mass of magnesium=mass of oxygen in oxide product The empirical formula of the oxide…arrow_forwardA chemist hoped to make an ethyl ester using diazoethane. At the end of the reaction, only the original carboxylic acid, ethene, and nitrogen were produced: □ CH H EXP 0+ H₂C OH Suggest an arrow-pushing mechanism that accounts for these products. Draw all missing reactants and/or products in the appropriate boxes by placing atoms on the canvas and connecting them with bonds. Add charges where needed. Electron-flow arrows should start on the electron(s) of an atom or a bond and should end on an atom, bond, or location where a new bond should be created. L CONT. 1 H₂C O NEN: OH ΝΕΝ OH *O* + H₂C=CH₂ + N₂ H с N O S CI Br Iarrow_forwardClassify each chemical reaction.arrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY