
Chemistry
10th Edition
ISBN: 9781305957404
Author: Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Concept explainers
Question
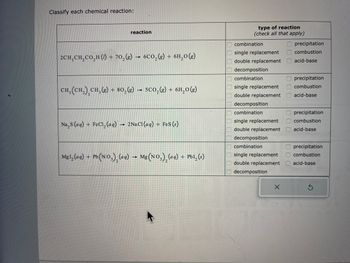
Transcribed Image Text:Classify each chemical reaction:
reaction
2CH₂ CH₂CO₂H (1) + 70₂(g) → 6CO₂(g) + 6H₂0(g)
CH, (CH₂), CH₂ (8) + 80₂(g) → 5CO₂(g) + 6H₂0 (g)
Na₂S (aq) + FeCl₂ (aq) → 2NaCl(aq) + FeS (s)
Mg1₂ (aq) + Pb (NO₂)₂ (aqg) → Mg(NO3)₂ (aq) + PbI₂ (s)
type of reaction
(check all that apply)
combination
single replacement
double replacement
decomposition
combination
single replacement
double replacement
decomposition
combination
single replacement
double replacement
decomposition
combination
single replacement
double replacement
decomposition
X
precipitation
combustion
acid-base
precipitation
combustion
acid-base
precipitation
combustion
acid-base
precipitation
combustion
acid-base
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by stepSolved in 3 steps with 3 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- One way in which the useful metal copper is produced is by dissolving the mineral azurite, which contains copper(II) carbonate, in concentrated sulfuric acid. The sulfuric acid reacts with the copper(II) carbonate to produce a blue solution of copper(II) sulfate. Scrap iron is then added to this solution, and pure copp metal precipitates out because of the following chemical reaction: Fe(s) + CUSO ¿(aq) → Cu(s) + FeSO,(aq) Suppose an industrial quality-control chemist analyzes a sample from a copper processing plant in the following way. He adds powdered iron to a 500. mL copper(II) sulfate sample from the plant until no more copper will precipitate. He then washes, dries, and weighs the precipitate, and finds that it has a mass c 97. mg. Calculate the original concentration of copper(II) sulfate in the sample. Round your answer to 2 significant digits.arrow_forwardSomeone is attempting to measure the H3PO4(aq) content of an unknown solution by both titration with NaOH(aq) and calorimetry. The person measures 100 mL of stock NaOH(aq) solution into a buret but forgets to write down its concentration. They then measure out 50.0 mL of unknown H3PO4(aq) solution (which has an indicator) and places this solution in a constant-pressure calorimeter for the titration experiment. The initial temperature of all the solutions is 22.9 Celsius. The student adds 46.84 mL of NaOH(aq) from the buret to the H3PO4(aq) solution in the calorimeter to reach the equivalence point (color change for indicator). The final solution temperature is 33.5 Celsius. Assume density and specific heat of all solutions are the same as that of pure water and that volumes are additive. a) what is the balanced molecular equation for the acid-base reaction that takes place in the calorimeter? b) Calculate delta HRXN (enthralpy) for the above acid-base reaction c) what is the…arrow_forwardAll of the following reactions can be described as displacement reactions except 3CuCl2(aq) + 2Al(s) → 3Cu(s) + 2AlCl3(aq). CH4(g) + Br2(g) → CH3Br(g) + HBr(g). Fe(s) + 2HCl(aq) → FeCl2(g) + H2(g). CuSO4(aq) + Ni(s) → NiSO4(aq) + Cu(s). Mg(s) + Hg(NO3)2(aq) → Mg(NO3)2(aq) + Hg(l). Provide explanation to given answer.arrow_forward
- Classify each chemical reaction: HI (aq) + NaOH(aq) 1 reaction Nal (aq) + H₂O(1) Na₂CO3 (s) → Na₂O(s) + CO₂(g) CuSO4 (aq) + Zn CrO (aq) ZnSO4 (aq) + CuCrO4(s) NaCl(aq) + AgNO3(aq) → NaNO, (aq) + AgCl(s) type of reaction (check all that apply) combination single replacement double replacement decomposition combination single replacement double replacement decomposition combination single replacement double replacement decomposition combination single replacement double replacement decomposition X precipitation combustion acid-base precipitation combustion acid-base precipitation combustion acid-base precipitation combustion acid-base Śarrow_forwardFor each chemical reaction listed in the table below, decide whether the highlighted atom is being oxidized or reduced. CH4(9)+H₂O(g) → CO(g) + 3 H₂(g) Na₂CO3(s) + H₂PO4(aq) → Na₂HPO4(aq) + CO₂(g) + H₂O(1) 2H,S(aq)+O,(g) →2S(s)+2H,O) FeO(s)+CO(g) → Fe(s)+CO₂(g) Explanation reaction Check highlighted atom is being... oxidized O O O O reduced οιοιοιο O X neither oxidized nor reduced MacBook Air O O S © 2023 McGraw Hill LLC. All Rights Reserved. Terms of U=arrow_forwardSodium hypochlorite, NACIO, is the active ingredient in household bleach The concentration of the hypochlorite ion, CIO, can be determined in two stages. In the first stage, a potassium iodide solution which has been acidified, is added to a solution of the bleach and iodine was formed. ClO (aq)+H(aq) +I(aq) L(aq) + Cl(aq) + H,OD In the second stage, the iodine formed was titrated with sodiumm thiosulphate solution. S,0, (aq) +I,(aq)21(aq) + SO6 (aq) 10.0 cm of a household bleach was diluted to 250cm in a standard flask thereafter 25 cm' of this solution was added to excess acidified potassium iodide solution. The solution was then titrated with 0.10 mol.L Sodium thiosulphate using an appropriate indicator. The volume of thiosulphate solution required to reach the end point of the titration was 20,5cm (a) Calculate the number of moles of Iodine which reacted in the titration (six decimal places only ie., 0.000001) mol (b) Calculate the concentration, in mol L, of Clo in the original…arrow_forward
- When the following molecular equation is balanced using the smallest possible integer coefficients, the values of these coefficients are: CH4 (g) + CCI4 (g) →→→ CH₂Cl₂ (g)arrow_forwardOne way in which the useful metal copper is produced is by dissolving the mineral azurite, which contains copper(II) carbonate, in concentrated sulfuric acid. The sulfuric acid reacts with the copper(II) carbonate to produce a blue solution of copper(II) sulfate. Scrap iron is then added to this solution, and pure copper metal precipitates out because of the following chemical reaction: Fe(s) + CuSO (aq) → Cu(s) + FeSO4(aq) ? Suppose an industrial quality-control chemist analyzes a sample from a copper processing plant in the following way. He adds powdered iron to a 150. mL copper(II) sulfate sample from the plant until no more copper will precipitate. He then washes, dries, and weighs the precipitate, and finds that it has a mass of 89. mg. 00. Calculate the original concentration of copper(II) sulfate in the sample. Round your answer to 2 significant digits. 0 || 09 Explanation Check x10 X G 2022 McGraw Hill LLC. All Rights Reserved. Terms of Use | Privacy Center | Accessibility Ararrow_forwardClassify each chemical reaction: Reaction Zn(s) + FeSO₂ (aq) → ZnSO₂ (aq) + Fe(s) CuSO₂ (aq) + ZnCrO₂ (aq) → ZnSO₂ (aq) + CuCrO₂ (s) - 2 Na(s) + Cl₂(g) → 2NaCl(s) 2H₂O₂(1)→ 2H₂O() + 0₂ (8) Type ✓ choose one combination decomposition single substitution double displacement none of the above choose onearrow_forward
- For each chemical reaction listed in the table below, decide whether the highlighted atom is being oxidized or reduced. reaction H₂S(aq) + 2NaOH(aq) → Na₂S(aq) + 2 H₂O(1) FeO(s)+CO(g) → Fe(s) + CO₂(g) CO(g) + H₂O(g) → CO₂(g) + H₂(g) 2 NH3(aq) + H₂SO4(aq) → (NH4)2SO4(aq) highlighted atom is being... oxidized reduced O O O X neither oxidized nor reduced 5arrow_forwardClassify each chemical reaction: KOH(aq) + HBrO (aq) reaction K BrO (aq) + H₂O (1) Na Cl (aq) + AgNO3(aq) → NaNO3(aq) + Ag Cl (s) 16K (s) + S₂ (s)→ 8K₂S (s) 2CH₂CH₂CO₂H (1) + 70₂(g) → 6CO₂(g) + 6H₂O(g) ✓ 6 ✓ 7 type of reaction (check all that apply) combination single replacement double replacement decomposition combination single replacement double replacement decomposition combination single replacement double replacement decomposition combination single replacement double replacement decomposition ✓8 X precipitation combustion acid-base precipitation combustion acid-base precipitation combustion acid-base precipitation combustion acid-base 9 10arrow_forwardPennies that are currently being minted are composed of zinc coated with copper. A student determines the mass of a penny to be 2.482 g and then makes several scratches in the copper coating (to expose the underlying zinc). The student puts the scratched penny in hydrochloric acid, where the following reaction occurs between the zinc and the HCl (the copper remains undissolved): Zn(s) + 2 HCl(aq) --------> H2(g) + ZnCl2(aq)The student collects the hydrogen produced over water at 25°C. The collected gas occupies a volume of 0.899 L at a total pressure of 791 mmHg. Calculate the percent zinc (by mass) in the penny. (Assume that all the Zn in the penny dissolves.)arrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY