
Chemistry
10th Edition
ISBN: 9781305957404
Author: Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Question
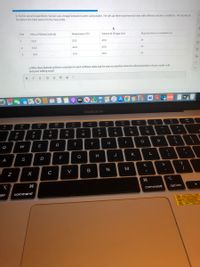
Transcribed Image Text:2. For his second experiment, Samuel uses vinegar instead of acetic acid powder. He sets up three experimental trials with different reaction conditions. He records all
his data in the table below for the three trials.
Trial
Mass of Baking Soda (g)
Temperature (°C)
Volume of Vinegar (mL)
Reaction time to completion (s)
1
10.0
25.0
60.0
41
10.0
40.0
60.0
23
3
10.0
55.0
60.0
12
c) Why does Samuel achieve a reaction in each of these trials, but he saw no reaction when he mixed powders of pure acetic acid
and pure baking soda?
BIUE E E
Σ
Aa
2048
tv
MacBook Air
F10
F4
FS
F1
F2
&
@
23
8
2
3
4
E
R
Y
U
Q
W
G
H
J
K
A
S
D
V
B
N
M
с
command
option
Fon
command
.. .-
N
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution
Trending nowThis is a popular solution!
Step by stepSolved in 2 steps

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- A solid mixture consists of 32.3 g of KNO3 (potassium nitrate) and 5.7 g of K2SO4 (potassium sulfate). The mixture is added to 130. g of water. If the solution described in the introduction is cooled to 0°C, what mass of KNO3 should crystallize? Enter your answer numerically in grams. Use this solubility curve (Figure 1) to answer the questions. • View Available Hint(s) ΑΣφ ? 0.0 Submit Previous Answersarrow_forwardYou are asked to make 43.0 grams of iron (Fe) from iron III oxide (Fe-Os) and carbon monoxide (CO) as shown in the chemical equation below. Fe₂O3 + 3C0 →→ 2Fe + 3C0₂2 How many grams of iron (III) oxide must you use? 0 A 61.5 g B. 122 g C. 0.777 g D. 686 garrow_forwardCompound A can be converted to either B or C. The energy diagrams for both processes are drawn on the graph below. Select the single best answer for each part. Energy Part 1 of 2 D E Reaction coordinate Label the A →→ C reaction as endothermic or exothermic. Endothermic Exothermic B X Ś 8 B olo 18 Ararrow_forward
- A precipitation forms when solutions of lead (II) nitrate and potassium iodide are mixed. Write the Net lonic Equation for this reaction. Your answer must contain the correct subscripts and superscripts. HTML Editor BI U A A I E E E E x' x, E I T 12pt Paragrapharrow_forward2. For his second experiment, Samuel uses vinegar instead of acetic acid powder. He sets up three experimental trials with different reaction conditions. He records all his data in the table below for the three trials. Trial Mass of Baking Soda (g) Temperature (°C) AVolume of Vinegar (mL) Reaction time to completion (s) 1 10.0 25.0 60.0 41 2 10.0 40.0 60.0 23 3 10.0 55.0 60.0 12 a) Describe a pattern you observe in Samuel's experimental results. In your answer, cite specific data from the table above. BIYE E m Σ 0/ 10000 Word Limit Aa 2048 26 C37.006 tv MacBook Air B88 F10 FS F4 & 23 %3D 4 5 6 8 2 3 { T Y Q W E G H J K A S D M V command option command . .. R Narrow_forwardimagine that a 2.0 m colution of copper 2 chloride is added to a piece of aluminum. draw a particulate representation of the mixture after any reaction has completedarrow_forward
- You are given 500 mL of a 0.50 M solution of MgCl₂. What is the theoretical yield (g) of Mg metal we could extract from the solution? a. 28g b. 24.3g c. 6.1g d 97.2 g e. 12.2 g Next Farrow_forwardConverting Concentrations to Different Units Introduction: Depending on how the concentration unit is defined, concentration can serve as a conversion between the amount of a solute and the amount of a solution or solvent. Concentrations can therefore be helpful in a number of stoichiometry issues. The original definition of the concentration unit is frequently preferred because it contains the conversion factor. Today, for chemists, it is suitable to make a solution in the lab using a specific concentration unit like molarity, and then convert the concentration to another unit. Today in the lab, I will prepare a sodium bicarbonate (baking soda) solution with a specific molarity. I will then translate the concentration into molality, mass percent, and mole fraction. Procedure The first step of this lab was to make sure I had everything I needed and that my surroundings were safe. I then looked at an empty beaker weighing paper and a balance. I first took a piece of weighing…arrow_forwardWhat occurs when aqueous lithium hydroxide, LiOH, reacts with aqueous magnesium chloride, MgCl2 ? Select one: O LIOH forms as a precipitate. O MgCl, forms as a precipitate. O No precipitate forms and no reaction occurs. O Mg(OH)2 forms as a precipitate O LICI forms as a precipitate.arrow_forward
- 13. Nitric acid, HNO3, is a strong acid in aqueous solutions. It is sold commercially as a concentrated solution with a density of 2.14 kg.L-¹ and containing 58% mass of HNO3. We introduce 10.0 mL of this solution in a 250.00 mL volumetric flask which is then filled to the mark with distilled water. This solution is called S. S is then diluted 100 times to afford solution S'. a. What is the definition of a strong acid? | b. What is the concentration of solution S"? c. What is the pH of solution S'?arrow_forwardChoose all of the given components that should not be included in a Materials and Methods/Experimental section of a research article. tables of data identifying the amount of product formed by chemical reactions being studied the claims that the author wants to make regarding the research the conclusions that can be made from the data taken in the research project the types of instruments and devices used to conduct the research why the particular instruments were used to conduct the research tables of data identifying the amount of each reactant in the research project figures that include the data collected in the research project O O Oarrow_forwardWhen solutions of silver nitrate and sodium phosphate are mixed a precipitate will form. Write the Total lonic Equation for this reaction. Your answer must contain the correct subscripts and superscripts. HTML Editor B I UA I E E E E E x x, E I T 12pt Paragrapharrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY