
Chemistry
10th Edition
ISBN: 9781305957404
Author: Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Concept explainers
Question
Pls I need this fast in 10 min thank you
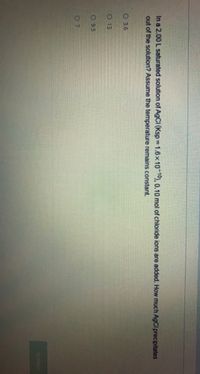
Transcribed Image Text:In a 2.00 L saturated solution of AGCI (Ksp 1.6x10 10), 0.10 mol of chloride ions are added. How much AgCl precipitates
out of the solution? Assume the temperature remains constant.
O 3.6
O 13
O 9.5
SUDMIT
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution
Trending nowThis is a popular solution!
Step by stepSolved in 3 steps

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- All changes save 7. When two solutions are mixed, a color change occurs. The data tables show the time between mixing and the color change for two sets of conditions. For Condition One, the solution concentrations were constant and temperature varied. For Condition Two, the temperature was constant and concentrations varied. Condition One: Concentration Time for Temperature (°C) Sample Color to Change 1 10° 36 sec 22° 14 sec Condition Two: Temperature Time for Concentration Sample Color to % Change 1. 100% 15 sec 2. 50% 24 sec Which of these statements is true according to the data? O The reaction rate is greater at 22°C than at 10°C. O Reducing the temperature increases the rate of the reaction. The reaction is affected by changes in temperature not by changes in concentration. O Decreasing the concentration increases the reaction rate. PREVIOUS 17 of 25 NEXT SAVE & EXITarrow_forwardKindly answer question d & earrow_forwardhrome File Edit View History Bookmarks Profiles Tab Window Help 令Q 2 O Mon Apr 25 Watch Gilmore Girls | Ne x a ALEKS A ALEKS - Reyna Garcia A ALEKS - David Teague 9,511 Jobs in Wilmington x O New Tab A www-awu.aleks.com/alekscgi/x/lsl.exe/1o_u-lgNslkr7j8P3jH-lvdWKW BBZZ16tTytly4Fcfu6zOtOf8oMM9sQvs3exlZv5th3sCgLJcZoF3sZYH-. O R Paused O Spotify Web Playe.. M Common Ethical D.. 国 Objective Knowledge Check Question 11 Reyna V Aqueous hydrochloric acid (HCl) will react with solid sodium hydroxide (NaOH) to produce aqueous sodium chloride (NaCl) and liquid water (H,O). Suppose 4.37 g of hydrochloric acid is mixed with 7.0 g of sodium hydroxide. Calculate the maximum mass of sodium chloride that could be produced by the chemical reaction. Be sure your answer has the correct number of significant digits. 圖 dlo IIarrow_forward
- Edit View History Bookmarks People Tab Window Help O Questi x L 2021-0 x M [EXTE! X Dashb x O Launc O Launcix S Class eto.mheducation.com/ext/map/index.html?_con%3con&external_browser=0&launchUrl=https%253A%252F%252 20 Problem Set Saved attempts left Check my work Enter your answer in the provided box. Find AG for the following reaction, at 25°C, using AH and S values. f NHẠCI(s) → NH3(g) + HCI(g) kJ Standard Thermodynamic Values at 298 K Substance or Ion AH (kJ/mol) (J/mol K) HCI(g) HCI(aq) NH3(g) • NH3(aq) NH,CI(s) -92.3 186.79 55.06 -167.46 -45.9 193 -80.83 110 -314.4 94.6 ( Prey 6 of 15arrow_forwardHelp 100% 47 T. "ublic Health Ch HSC 258 - Major Projec x MindTap - Cengage Lea X C The Illustration To T =55750828934189288909969212&elSBN=9781305657571&id=D1061392007&nbld=21... * Q Search t Referonces Use the References to access important values if needed for this question. For the following reaction, 50.4 grams of sulfur dioxide are allowed to react with 17.9 grams of water. sulfur dioxide (g) + water (I) sulfurous acid (H2SO3) (g) grams What is the maximum amount of sulfurous acid (H,SO3) that can be formed? What is the FORMULA for the limiting reagent? grams What amount of the excess reagent remains after the reaction is complete? Submit Answerarrow_forwardIn analytical instrument the stimulus can be digitizer or plotter. O True O Falsearrow_forward
- Paraphrasing Tool | QuillBot AI X b Answered: Part A Classify each x Course Home Dashboard UC 6 Yoga Asanas To Help You Bur X openvellum.ecollege.com/course.html?courseld=16519516&OpenVellumHMAC=6acab9256cb89b6deOb7314be966bc5a#10001 Apps Yahoo Mail YouTube Maps Best Free PowerP... Google Drive on Academic Search Downloads € University Librarie... E UNIVERSITY POR... Student Detail Sc... > I Review I Constants I Periodic Table Scores Balance each of the following by determining coefficients, and identify the type of reaction. eТext Part A Document Sharing Identify the coefficients in the reaction: User Settings Course Tools > Enter your answers in order from left to right numerically separated by commas. Use the lowest possible coefficients. ? Submit Request Answer Part B Identify the type of reaction in Part A. combination double replacement combustion decomposition P Pearson Copyright © 2021 Pearson Education Inc. All rights reserved. Terms of Use | Privacy Policy | Permissions |…arrow_forwardA certified reference material of lead in calcium carbonate is analyzed by a laboratory and found to have a lead content of 257 ng/g. The reported certified value for this material is 235 ng/g. What is the absolute error and the percent relative error for the method used in this analysis? absolute error: percent relative error: ng/g %arrow_forwardI don't understand How to do this question and I am on my last try please help me!arrow_forward
- 5. A student mixed 5 mL of 3% m/v H202 with 10 mL of 0.5M IKI solution and measured the volume of water displaced over time. Use their data to prepare a plot of volume water displaced (mL) versus time (s) in Excel and find the rate of this run. Attach your spreadsheet and plot to your pre-laboratory assignment. Volume of Water Displaced (mL) Time (s) 2.0 39 4.0 76 6.0 106 8.0 137 10.0 166 12.0 192 14.0 222 16.0 242 18.0 20.0 270 302 Rate =arrow_forwardConter Import favorites Conter + Cr³+ (aq) + 3 e Zn²+ (aq) + 2 e 13.1 CH Ak X 49°F Light rain 07 Detern https://app.101edu.co a Amazon.com - Onli... Home hp Booking.com After 3 Q Which QWrite a a Consid LastPass password... Q Search Cr(s) Eºred = -0.744 V → Zn(s) E°red = -0.763 V Consider a galvanic electrochemical cell constructed using Cr/Cr³+ and Zn/Zn²+ at 25 °C. The following half-reactions are provided for each metal: Compare Wrapsod... Time's Up! Given that the standard cell potential is 0.019 V and the overall equation is 2 Cr³+ (aq) + 3 Zn (s) → 2 Cr (s) + 3 Zn²+ (aq), what is the cell potential for this cell at 25°C when [Zn²+] = 0.0143 M and [Cr] 0.1556 M? W Q Cr³(aq Q hp TurboTax Deluxe 20... mylip Given t C Consid = Q 1 +/- 1 The University of Ve... a Consic b Answe C Consic + 2 LO 5 8 V 3 6 9 0 Other favorites Submit X C X 100 t May 4, 2023, 12:37 AM 12:37 A 5/4/20arrow_forwardPls solve this question correctly instantly in 5 min i will give u 3 like for surearrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY