
Pushing Electrons
4th Edition
ISBN: 9781133951889
Author: Weeks, Daniel P.
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Concept explainers
Question
Determine the
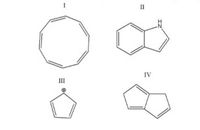
Transcribed Image Text:II
IV
II
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by stepSolved in 3 steps with 3 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- Draw the major organic product of each reaction. Assume a one-to-one ratio of reagents and benzene. For the functional groups added. be sure to draw out all bonds and formal charges. 1st attempt Part 1 iSee Periodic Table ONH conc. H, SO,arrow_forwardBr Bri 1 equiv. NaN3 DMSOarrow_forward1.76 How does Gorilla Glass differ from more commonly found alumina silicate glass?arrow_forward
- Hexane (C6H14, density = 0.766 g/cm3), perfluoro-hexane (C6F14, density = 1.669 g/cm3), and water are immiscible liquids; that is, they do not dissolve in one another. You place 10 mL of each in a graduated cylinder, along with pieces of high-density polyethylene (HDPE, density = 0.97 g/cm3), polyvinyl chloride(PVC, density = 1.36 g/cm3), and Teflon (density = 2.3 g/cm3). None of these common plastics dissolves in these liquids. Describe what you expect to see.arrow_forward2 equiv. Η ΘΗarrow_forward7. From the normal curve of error, find the probability that a result is outside the limits of ±2σ from the mean. What is the probability that a result has a more negative deviation from the mean than -2σ?arrow_forward
- 1. 2 Li, Et,0 2. 0.5 equiv. Cul Br 3. CH3(CH2)2Br FEB 20 étv MacBook Airarrow_forward4/3 pie * (0.0576/2)^3=0.00010061=1.00=1.00x2.20/60.02=0.036654449*60.09=2.202565811=2.2E-18 ? is this even right? i've been struggling with this problem for hours.arrow_forwardEvery year Every second (1 year 365 days).arrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you

 Chemistry for Engineering StudentsChemistryISBN:9781337398909Author:Lawrence S. Brown, Tom HolmePublisher:Cengage Learning
Chemistry for Engineering StudentsChemistryISBN:9781337398909Author:Lawrence S. Brown, Tom HolmePublisher:Cengage Learning Chemistry & Chemical ReactivityChemistryISBN:9781337399074Author:John C. Kotz, Paul M. Treichel, John Townsend, David TreichelPublisher:Cengage Learning
Chemistry & Chemical ReactivityChemistryISBN:9781337399074Author:John C. Kotz, Paul M. Treichel, John Townsend, David TreichelPublisher:Cengage Learning Chemistry & Chemical ReactivityChemistryISBN:9781133949640Author:John C. Kotz, Paul M. Treichel, John Townsend, David TreichelPublisher:Cengage Learning
Chemistry & Chemical ReactivityChemistryISBN:9781133949640Author:John C. Kotz, Paul M. Treichel, John Townsend, David TreichelPublisher:Cengage Learning Chemistry for Engineering StudentsChemistryISBN:9781285199023Author:Lawrence S. Brown, Tom HolmePublisher:Cengage Learning
Chemistry for Engineering StudentsChemistryISBN:9781285199023Author:Lawrence S. Brown, Tom HolmePublisher:Cengage Learning


Chemistry for Engineering Students
Chemistry
ISBN:9781337398909
Author:Lawrence S. Brown, Tom Holme
Publisher:Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:9781337399074
Author:John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:9781133949640
Author:John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:Cengage Learning

Chemistry for Engineering Students
Chemistry
ISBN:9781285199023
Author:Lawrence S. Brown, Tom Holme
Publisher:Cengage Learning
