
Biochemistry
9th Edition
ISBN: 9781319114671
Author: Lubert Stryer, Jeremy M. Berg, John L. Tymoczko, Gregory J. Gatto Jr.
Publisher: W. H. Freeman
expand_more
expand_more
format_list_bulleted
Question
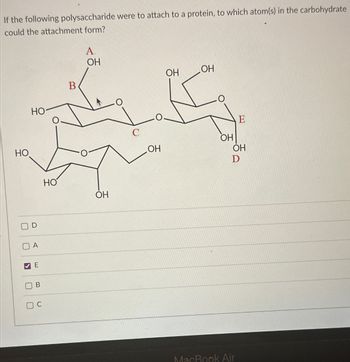
Transcribed Image Text:If the following polysaccharide were to attach to a protein, to which atom(s) in the carbohydrate
could the attachment form?
HO
U
☐
☐
☑
D
A
E
HO
B
B
C
A
OH
OH
OH
HO
OH
E
OH
OH
D
وة
OH
MacBook Air
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by stepSolved in 3 steps

Knowledge Booster
Similar questions
- Select the incorrect statement(s). CH₂ OH NH₂ Polypeptide backbone CH₂ A C- CH₂ CH CH3 CH3 CH3 CH3 CH CH₂ NH₂ CH₂ CH₂ CH₂ CH₂ B D This is a tertiary structure of a protein. Letter A represents hydrogen bond. Letter C represents disulfide bond. This represents a secondary structure. This illustrates a quaternary structure.arrow_forwardPlease don't provide handwriting solutionarrow_forwardWe are consuming a lot of polymers in our foods and only some monomers. The polymers we ingest must be converted into monomers before being absorbed, with a few exceptions that we won't discuss. Amylase fatty acid nucleotide amino acid ribonuclease peptide polysaccharide triglyceride trypsin monosaccharide RNA lipase Fill the table. Ise the word bank above to fill-in the columns: macronutrient polymer Enzyme that hydrolyzes polymer into monomer monomer Proteins amino acid Carbohydrates monosaccharide Lipids fatty acid Nucleic acidsarrow_forward
- How do I combine these three amino acids into a tripeptide?arrow_forwardWhich of the following most directly applies to the formation of the secondary structures of proteins? A B с D formation of ionic bonds between the R groups of two polypeptides formation of nonpolar interactions between two R groups on the same polypeptide formation of covalent bonds via dehydration reactions between two amino acids formation of hydrogen bonds between between amino and carboxyl groupsarrow_forwardThe image below represents three monomers of a polysaccharide chain. Which polysaccharide does this represent? ÇH,OH Om ÇH,OH Он OH он OHarrow_forward
- Classify the following samples as amino acid, peptide, or protein.arrow_forwardCan someone explain thisarrow_forwardUV-vis spectroscopy can be used to determine protein and nucleic acid concentrations, provided the user knows the molar extinction coefficient of the protein or nucleic acid. Rank the wavelength absorption for the amino acids below from shortest wavelength to longest in the UV region.arrow_forward
- The following is an example of which level of protein structure? What bonds form this level of structure? Asp-Met-Leu-Trp-Gly-Asn-Lysarrow_forwardI'd like you to explain to me the structure of one of your macromolecules. You should be describing the monomers, polymers, and any important chemical bonding. As an example polenta carbohydrates.arrow_forwardPenicillin G is a natural antibiotic that is useful for treating infections caused by Gram positive bacteria. What is the functional benefit of the semi-synthetic antimicrobials carbenicillin and ampicillin, generated by chemical modification of the R group so their R groups each are a bit different than the R group seen with penicillin G?arrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 BiochemistryBiochemistryISBN:9781319114671Author:Lubert Stryer, Jeremy M. Berg, John L. Tymoczko, Gregory J. Gatto Jr.Publisher:W. H. Freeman
BiochemistryBiochemistryISBN:9781319114671Author:Lubert Stryer, Jeremy M. Berg, John L. Tymoczko, Gregory J. Gatto Jr.Publisher:W. H. Freeman Lehninger Principles of BiochemistryBiochemistryISBN:9781464126116Author:David L. Nelson, Michael M. CoxPublisher:W. H. Freeman
Lehninger Principles of BiochemistryBiochemistryISBN:9781464126116Author:David L. Nelson, Michael M. CoxPublisher:W. H. Freeman Fundamentals of Biochemistry: Life at the Molecul...BiochemistryISBN:9781118918401Author:Donald Voet, Judith G. Voet, Charlotte W. PrattPublisher:WILEY
Fundamentals of Biochemistry: Life at the Molecul...BiochemistryISBN:9781118918401Author:Donald Voet, Judith G. Voet, Charlotte W. PrattPublisher:WILEY BiochemistryBiochemistryISBN:9781305961135Author:Mary K. Campbell, Shawn O. Farrell, Owen M. McDougalPublisher:Cengage Learning
BiochemistryBiochemistryISBN:9781305961135Author:Mary K. Campbell, Shawn O. Farrell, Owen M. McDougalPublisher:Cengage Learning BiochemistryBiochemistryISBN:9781305577206Author:Reginald H. Garrett, Charles M. GrishamPublisher:Cengage Learning
BiochemistryBiochemistryISBN:9781305577206Author:Reginald H. Garrett, Charles M. GrishamPublisher:Cengage Learning Fundamentals of General, Organic, and Biological ...BiochemistryISBN:9780134015187Author:John E. McMurry, David S. Ballantine, Carl A. Hoeger, Virginia E. PetersonPublisher:PEARSON
Fundamentals of General, Organic, and Biological ...BiochemistryISBN:9780134015187Author:John E. McMurry, David S. Ballantine, Carl A. Hoeger, Virginia E. PetersonPublisher:PEARSON

Biochemistry
Biochemistry
ISBN:9781319114671
Author:Lubert Stryer, Jeremy M. Berg, John L. Tymoczko, Gregory J. Gatto Jr.
Publisher:W. H. Freeman

Lehninger Principles of Biochemistry
Biochemistry
ISBN:9781464126116
Author:David L. Nelson, Michael M. Cox
Publisher:W. H. Freeman

Fundamentals of Biochemistry: Life at the Molecul...
Biochemistry
ISBN:9781118918401
Author:Donald Voet, Judith G. Voet, Charlotte W. Pratt
Publisher:WILEY

Biochemistry
Biochemistry
ISBN:9781305961135
Author:Mary K. Campbell, Shawn O. Farrell, Owen M. McDougal
Publisher:Cengage Learning

Biochemistry
Biochemistry
ISBN:9781305577206
Author:Reginald H. Garrett, Charles M. Grisham
Publisher:Cengage Learning

Fundamentals of General, Organic, and Biological ...
Biochemistry
ISBN:9780134015187
Author:John E. McMurry, David S. Ballantine, Carl A. Hoeger, Virginia E. Peterson
Publisher:PEARSON