
Biochemistry
9th Edition
ISBN: 9781319114671
Author: Lubert Stryer, Jeremy M. Berg, John L. Tymoczko, Gregory J. Gatto Jr.
Publisher: W. H. Freeman
expand_more
expand_more
format_list_bulleted
Question
Solve correctly please.
Should correct
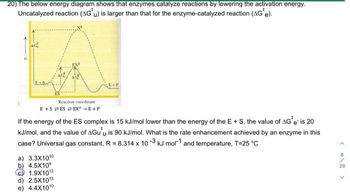
Transcribed Image Text:20) The below energy diagram shows that enzymes catalyze reactions by lowering the activation energy.
Uncatalyzed reaction (AG'u) is larger than that for the enzyme-catalyzed reaction (AG*e).
AG
a) 3.3X1010
b) 4.5X109
EXI
Reaction coordinate
E+SES EXE+P
1.9X10¹2
d) 2.5X10¹3
e) 4.4X1010
AG
If the energy of the ES complex is 15 kJ/mol lower than the energy of the E + S, the value of AG* e' is 20
kJ/mol, and the value of AGu u is 90 kJ/mol. What is the rate enhancement achieved by an enzyme in this
case? Universal gas constant, R = 8.314 x 10-3 kJ mol-1 and temperature, T=25 °C
<
29
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by stepSolved in 3 steps

Knowledge Booster
Similar questions
- Please ASAP. Thankyou. Please provide brief explanation.arrow_forwardHi I hope you're doing fine. I need help with these questions, please! thank you!arrow_forwardPlease help and make it short. Also, please don't use advanced words when explaining, thanks! Explain how the mRNA vaccine work and relate to protein synthesis (Moderna and Pfizer vaccines are both using this technology).arrow_forward
- Please ASAP. Thank you.arrow_forwardPlease don't copy. Give me correct answer.arrow_forwardAll the Bold answers are wrong please explain why it is wrong and give me the correct answer. Thanks, in advanced Which of the following is true about enzymes? Enzymes are equally effective across broad ranges of temperature and Ph. Enzymes lower the overall free energy of a reaction to make it spontaneous. Enzyme activity is generally unregulated. Enzymes recognize many substrates with equal specificity. The insulin receptor catalyzes the phosphorylation of several substrates and is therefore classified as a Transferase Phosphate Kinase Lyase Oxidoreductase 3)Which of the following reaction parameters can enzymes optimize to increase reaction rate? The proximity(=closeness) of the reacting groups. The rotational motions of the substrates and catalytic groups. The orientations of the substrates and catalytic groups. The achieve energy needed to achieve the transition state. The catalytic mechanism of RNA relies upon general acid-base catalysis involving the amino acid…arrow_forward
- Please asap. thanku If you gave someone a non-selective blocker of P2X channels,what functions would be impaired?arrow_forwardPlease ASAP. Thanku What is optogenetics? How does it work? what are its possible uses? Can we use this in humans, why? provide references.arrow_forwardPatent issues related to the drug Primaquine ?arrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 BiochemistryBiochemistryISBN:9781319114671Author:Lubert Stryer, Jeremy M. Berg, John L. Tymoczko, Gregory J. Gatto Jr.Publisher:W. H. Freeman
BiochemistryBiochemistryISBN:9781319114671Author:Lubert Stryer, Jeremy M. Berg, John L. Tymoczko, Gregory J. Gatto Jr.Publisher:W. H. Freeman Lehninger Principles of BiochemistryBiochemistryISBN:9781464126116Author:David L. Nelson, Michael M. CoxPublisher:W. H. Freeman
Lehninger Principles of BiochemistryBiochemistryISBN:9781464126116Author:David L. Nelson, Michael M. CoxPublisher:W. H. Freeman Fundamentals of Biochemistry: Life at the Molecul...BiochemistryISBN:9781118918401Author:Donald Voet, Judith G. Voet, Charlotte W. PrattPublisher:WILEY
Fundamentals of Biochemistry: Life at the Molecul...BiochemistryISBN:9781118918401Author:Donald Voet, Judith G. Voet, Charlotte W. PrattPublisher:WILEY BiochemistryBiochemistryISBN:9781305961135Author:Mary K. Campbell, Shawn O. Farrell, Owen M. McDougalPublisher:Cengage Learning
BiochemistryBiochemistryISBN:9781305961135Author:Mary K. Campbell, Shawn O. Farrell, Owen M. McDougalPublisher:Cengage Learning BiochemistryBiochemistryISBN:9781305577206Author:Reginald H. Garrett, Charles M. GrishamPublisher:Cengage Learning
BiochemistryBiochemistryISBN:9781305577206Author:Reginald H. Garrett, Charles M. GrishamPublisher:Cengage Learning Fundamentals of General, Organic, and Biological ...BiochemistryISBN:9780134015187Author:John E. McMurry, David S. Ballantine, Carl A. Hoeger, Virginia E. PetersonPublisher:PEARSON
Fundamentals of General, Organic, and Biological ...BiochemistryISBN:9780134015187Author:John E. McMurry, David S. Ballantine, Carl A. Hoeger, Virginia E. PetersonPublisher:PEARSON

Biochemistry
Biochemistry
ISBN:9781319114671
Author:Lubert Stryer, Jeremy M. Berg, John L. Tymoczko, Gregory J. Gatto Jr.
Publisher:W. H. Freeman

Lehninger Principles of Biochemistry
Biochemistry
ISBN:9781464126116
Author:David L. Nelson, Michael M. Cox
Publisher:W. H. Freeman

Fundamentals of Biochemistry: Life at the Molecul...
Biochemistry
ISBN:9781118918401
Author:Donald Voet, Judith G. Voet, Charlotte W. Pratt
Publisher:WILEY

Biochemistry
Biochemistry
ISBN:9781305961135
Author:Mary K. Campbell, Shawn O. Farrell, Owen M. McDougal
Publisher:Cengage Learning

Biochemistry
Biochemistry
ISBN:9781305577206
Author:Reginald H. Garrett, Charles M. Grisham
Publisher:Cengage Learning

Fundamentals of General, Organic, and Biological ...
Biochemistry
ISBN:9780134015187
Author:John E. McMurry, David S. Ballantine, Carl A. Hoeger, Virginia E. Peterson
Publisher:PEARSON