If a mixture of solid nickel(II) oxide and 0.13000 M carbon monoxide is allowed to come to equilibrium at 1500 K, what will be the equilibrium concentration of CO2? Express your answer using two significant figures. ? [CO2) = M
If a mixture of solid nickel(II) oxide and 0.13000 M carbon monoxide is allowed to come to equilibrium at 1500 K, what will be the equilibrium concentration of CO2? Express your answer using two significant figures. ? [CO2) = M
Introduction to General, Organic and Biochemistry
11th Edition
ISBN:9781285869759
Author:Frederick A. Bettelheim, William H. Brown, Mary K. Campbell, Shawn O. Farrell, Omar Torres
Publisher:Frederick A. Bettelheim, William H. Brown, Mary K. Campbell, Shawn O. Farrell, Omar Torres
Chapter7: Reaction Rates And Chemical Equilibrium
Section: Chapter Questions
Problem 7.17P: 7-17 If a certain reaction takes 16 h to go to completion at 10°C, what temperature should we run it...
Related questions
Question
![If a mixture of solid nickel(Il) oxide and 0.13000 M carbon monoxide is allowed to come to equilibrium at 1500 K, what will be the
equilibrium concentration of CO2?
Express your answer using two significant figures.
?
[CO2] =
M
%3D](/v2/_next/image?url=https%3A%2F%2Fcontent.bartleby.com%2Fqna-images%2Fquestion%2F8bccc44d-7782-46f2-838e-c140c2dedd7b%2Ff49e082d-ba2a-4d0b-9c74-5312e08df157%2Fg1jk7fr_processed.png&w=3840&q=75)
Transcribed Image Text:If a mixture of solid nickel(Il) oxide and 0.13000 M carbon monoxide is allowed to come to equilibrium at 1500 K, what will be the
equilibrium concentration of CO2?
Express your answer using two significant figures.
?
[CO2] =
M
%3D

Transcribed Image Text:Consider the reaction:
Nio (s) + CO (9) = Ni (s) + CO2 (g)
K. =4000.0 at 1500 K
When calculating the answer, do not round to the
appropriate number of significant figures until the last
calculation step.
Expert Solution
Step 1
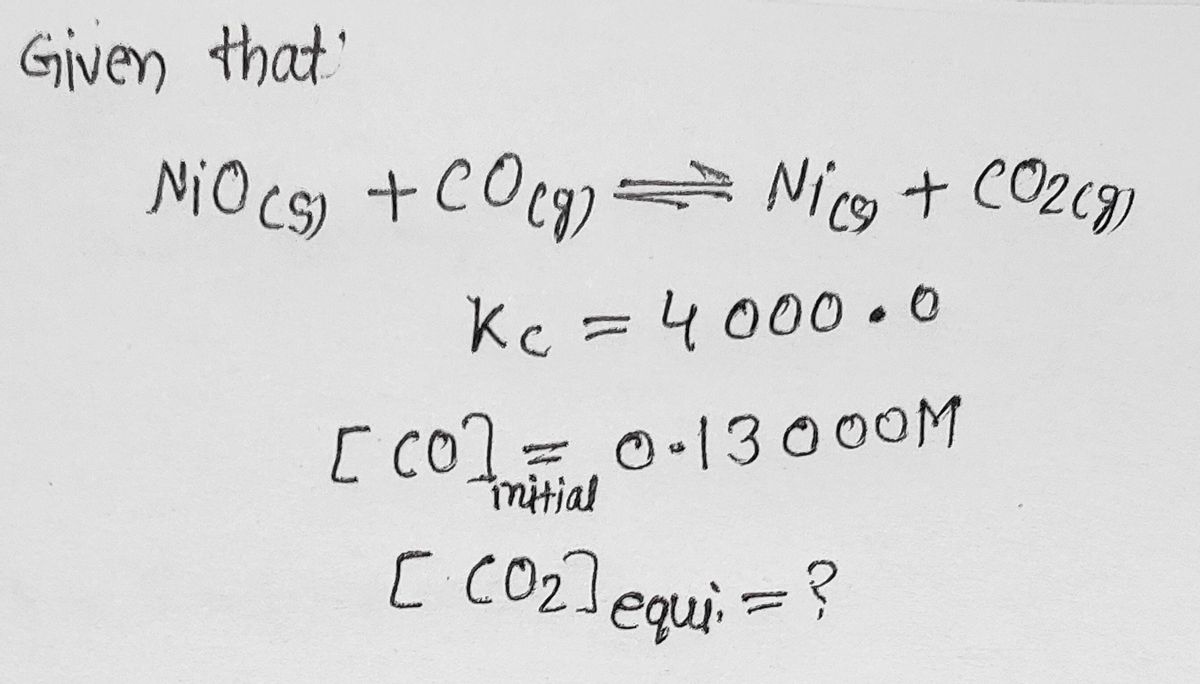
Trending now
This is a popular solution!
Step by step
Solved in 2 steps with 2 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

Introduction to General, Organic and Biochemistry
Chemistry
ISBN:
9781285869759
Author:
Frederick A. Bettelheim, William H. Brown, Mary K. Campbell, Shawn O. Farrell, Omar Torres
Publisher:
Cengage Learning


Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Introduction to General, Organic and Biochemistry
Chemistry
ISBN:
9781285869759
Author:
Frederick A. Bettelheim, William H. Brown, Mary K. Campbell, Shawn O. Farrell, Omar Torres
Publisher:
Cengage Learning


Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Chemistry: An Atoms First Approach
Chemistry
ISBN:
9781305079243
Author:
Steven S. Zumdahl, Susan A. Zumdahl
Publisher:
Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781337399074
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning

Chemistry: The Molecular Science
Chemistry
ISBN:
9781285199047
Author:
John W. Moore, Conrad L. Stanitski
Publisher:
Cengage Learning