
Chemistry
10th Edition
ISBN: 9781305957404
Author: Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Concept explainers
Question
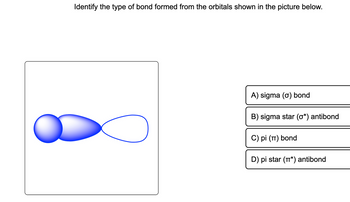
Transcribed Image Text:Identify the type of bond formed from the orbitals shown in the picture below.
A) sigma (o) bond
B) sigma star (0*) antibond
C) pi (π) bond
D) pi star (*) antibond
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution
Trending nowThis is a popular solution!
Step by stepSolved in 3 steps with 1 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- Can you please do the first 3 rows of the table?arrow_forwardI need help w/ everything thanksarrow_forwardNumber of nonbonding pairs: CH2O (C is central atom) Number of bonding groups: Total number of electron groups: Electron Geometry Molecular shape HCN (C is central atom) Number of nonbonding pairs: Number of bonding groups: Total number of electron groups: Electron Geometry Molecular shape Number of nonbonding pairs: CO,2 Number of bonding groups: Total number of electron groups: Electron Geometry Molecular shape PC13 Number of nonbonding pairs: Number of bonding groups: Total number of electron groups: Electron Geometry Molecular shapearrow_forward
- What are the angles a and b in the actual molecule of which this is a Lewis structure? H H H C H a a = C b = ⁰ C H Note for advanced students: give the ideal angles, and don't worry about small differences from the ideal that might be caused by the fact that different electron groups may have slightly different sizes. H Xarrow_forwardConsider the following ion: BrO3¯. a) Show the full electron configuration for Br. b) Draw the most correct Lewis structure for BrO3¯ and briefly explain why your Lewis structure is correct. c) If the structure is stabilised by resonance, draw at least one of the possible resonance forms. If it is not stabilised by resonance, briefly explain why. d) What is the electronic geometry of BrO3-? What is its molecular shape? e) Does BrO3 have a dipole moment? Briefly justify your answer. f) On average, would you expect IO3¯ to have longer or shorter bonds than BrO3¯? Briefly explain your answer. g) Which of the following molecules would you expect to have the lowest vapour pressure? Briefly explain your choice. Br HO HO. Br- Compound A Compound B Compound C h) What is the molecular formula for Compound C? What is the empirical formula for Compound C?arrow_forwardAnswer the following questions about the Lewis structure for the organic molecule CH3CH2CH2CN (see examples in Chapter 10) There are valence electrons. The molecule has single bonds, double bonds, and triple bonds. The three central C atoms on the left have REDS and the shape at each of these is The central C on the right has REDS and the shape is The AENC-N = 0.49 which is only slightly polar and we will consider it to be nonpolar. %3D Since there is only slightly polar covalent bond, the molecule is mostly а. О b. 1 С. 2 d. 3 е. 4 f. 5 g. 8 h. 10 i. 12 j. 14 k. 16 I. 18 m. 20 n. 24 О. 28 р. 30 q. 32 r. 34 S. 36 t. diatomic u. pyramidal v. linear w. monoatomic ions x. tetrahedral y. trigonal planar z. bent aa. 120° bb. 180° dd. 109.5° cc. no bond angles, no central atom nonpolar ее. polar ff. gg. ionic hh. C and Carrow_forward
- Draw a Lewis structure for the molecular formula: SnI3– This molecule only contains tin and iodine atoms. Only one atom in this molecule should have a nonzero formal charge. Has to show this nonzero formal charge in your Lewis structure. What is the geometry of the electron groups around iodine?arrow_forwardChoose the best Lewis structure for OCN. (It will help to work out the missing formal charges for the atoms in these different structures) [Image description: Lewis structure A has a C atom singly bound to a O atom and a N atom. There are three electron pairs on O and three electron pairs on N. Lewis structure B has a C atom singly bound to a O atom and triply bound to a N atom. There are three electron pairs on O and one electron pair on N. Lewis structure C has a C atom triply bound to a O atom and singly bound to a N atom. There is one electron pair on O and three electron pairs on N. Lewis structure D has a C atom doubly bound to a O atom and a N atom. There are two electron pairs on O and two electron pairs on N.] :0-c-N: :0-c=N: :0=C-N: o=c=N: A B Select an answer and submit. For keyboard navigation, use the up/down arrow keys to select an answer. a b В C C d Darrow_forward||| Predicting deviations from ideal bond angles Consider the perbromate (BrO4) : anion. What is the central atom? Enter its chemical symbol. How many lone pairs are around the central atom? What is the ideal angle between the bromine-oxygen bonds? Compared to the ideal angle, you would expect the actual angle between the bromine-oxygen bonds to be ... 0 口。 (choose one) (choose one) about the same bigger smallerarrow_forward
- Identify the molecular shape of each of the molecules. Molecule SiHA SFA LL F F-C-F |||| Geometry Molecule BrF5 PCI, Geometryarrow_forwardName Section Table 1: Complete this table with your partner. You may ask the instructor to check your work. Formula Lewis structure Molecular geometry and bond angle(s) around central atoms(s) Polar or nonpolar, draw dipole arrow Hybridization of each atom Orbitals involved in each bond CO2 2 x C=O: sp- sp o o=c=o° C: sp O: sp Linear, 180° Non-polar 2 x C=O: p-p TC COF2 CH2CH2 SCI2 SF4 atoms. atomarrow_forward
arrow_back_ios
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY