
Chemistry
10th Edition
ISBN: 9781305957404
Author: Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Concept explainers
Question
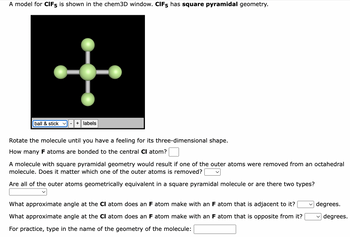
Transcribed Image Text:A model for CIF5 is shown in the chem3D window. CIF5 has square pyramidal geometry.
+
ball & stick
+ labels
Rotate the molecule until you have a feeling for its three-dimensional shape.
How many F atoms are bonded to the central CI atom?
A molecule with square pyramidal geometry would result if one of the outer atoms were removed from an octahedral
molecule. Does it matter which one of the outer atoms is removed?
Are all of the outer atoms geometrically equivalent in a square pyramidal molecule or are there two types?
What approximate angle at the CI atom does an F atom make with an F atom that is adjacent to it?
What approximate angle at the CI atom does an F atom make with an F atom that is opposite from it?
For practice, type in the name of the geometry of the molecule:
degrees.
degrees.
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution
Trending nowThis is a popular solution!
Step by stepSolved in 4 steps with 4 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- F. Electron-Dot Formulas and Shape Formula 1. Electron- Dot 2. Number of 3. Electron- 4. Number 5. Shape 6. Polar or Formula Electron Group Bonded Nonpolar? Groups Arrangement Atoms Tetrahedral H,0 Tetrahedral 2 Polar bent SF, Polar NI3 Polar SIB14 Nonpolar Nonpolar SO3 Nonpolar co2 D Focus 国 tearrow_forwardA model for BrF3 is shown in the Jmol window. BrF3 has T-shaped geometry. ball & stick ✓ + labels Rotate the molecule until you have a feeling for its three-dimensional shape. How many F atoms are bonded to the central Br atom? Are all of the positions about the central atom equivalent, or are there two kinds of positions? A T-shaped molecule can be derived from a trigonal bipyramidal molecule by removing two of the outer atoms. Would the atoms removed be both axial, both equatorial, or one of each? What approximate angle at the Br atom does the unique F atom make with either of the other two F atoms? For practice, type in the name of the geometry of the molecule: degrees. Previousarrow_forwardWhat is the electron geometry (EG) and molecular geometry (MG) of the compound H2S? Hint: Sulfur is in the same group as oxygen (6A). Group of answer choices EG = Tetrahedral MG = Tetrahedral EG = Trigonal planar MG = Trigonal planar EG = Tetrahedral MG = Trigonal pyramidal EG = Trigonal planar MG = Bent EG = Tetrahedral MG = Bentarrow_forward
- A Lewis structure is a two-dimensional representation of a molecule that does not necessarily show what shape that molecule would take in three dimensions. From the given Lewis structure and what you know about VSEPR theory, identify the shape of the molecule. XV-X tetrahedral bent linear T-shaped trigonal planar octahedral trigonal pyramidal square planar see-saw square pyramidal trigonal bipyramidalarrow_forwarda molecule is made from 4 atoms. the first atom (A) has 5 valence electrons, the second atom (B) has 1 valence electron, and the other two atoms (C) each have 7 valence electrons. what is the shape of the molecule ABC2?arrow_forwardDetermine whether each of the molecules below is polar or nonpolar. Linear F2 Tetrahedral SiF4 Linear CO Trigonal planar BCl3arrow_forward
- Consider the central atom in a molecule with electrons distributed in orbitals as shown below. From this, we know that the molecular geometry of the molecule is: ㄴ11 sp² sp² sp² P linear Otetrahedral OT-shaped O trigonal planar H Obentarrow_forwardMark the following statements as true or false. A molecule with very polar bonds can be nonpolar. blank The electrons in a polar bond are found nearer to the more electronegative element. blank Fluorine is very polarizable. blank Covalent bonds are partially ionic because of polarizability of atoms. blank Kr and Sr2- are isoelectronic.arrow_forwardVSEPR HOMEWORK Molecule Lewis Structure #e groups #lone-pair e Hybridization type VSEPR Formula (i.e Molecular Type) Geometric arrangement Molecular Shape Bond Angle Is the molecule Polar? Justify 3d Drawing PCI₂F₁arrow_forward
- VSEPR HOMEWORK Molecule Lewis Structure #e groups #lone-pair e Hybridization type VSEPR Formula (i.e Molecular Type) Geometric arrangement Molecular Shape Bond Angle Is the molecule Polar? Justify 3d Drawing CIF 3arrow_forwardUse the 3D structure below to determine whether BC33 is polar. To do this, you must use arrows to indicate polar bonds, and either use an arrow to indicate the net dipole moment or state that the molecule is non-polar.arrow_forward1. Consider the diatomic molecules ( molecules with 2 atoms ) in this exercise : O2, N2, Cl2, and HCl . Do you think a diatomic molecule can be any shape other than linear ? Explain . because They are only two alems 2. Look at the Lewis structure you drew for SCl2 and CS2 Both of these molecules are made up of three atoms but have different molecular geometries . a . How is the arrangement of electrons around the central atoms different ? Give the number of lone pairs and bonding groups around each central atom Based on your previous answer , explain why SCl2 can't be linear . c . Explain why one molecule is polar and the other is nonpolararrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY