
Chemistry
10th Edition
ISBN: 9781305957404
Author: Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Concept explainers
Question
Identify the mechanism
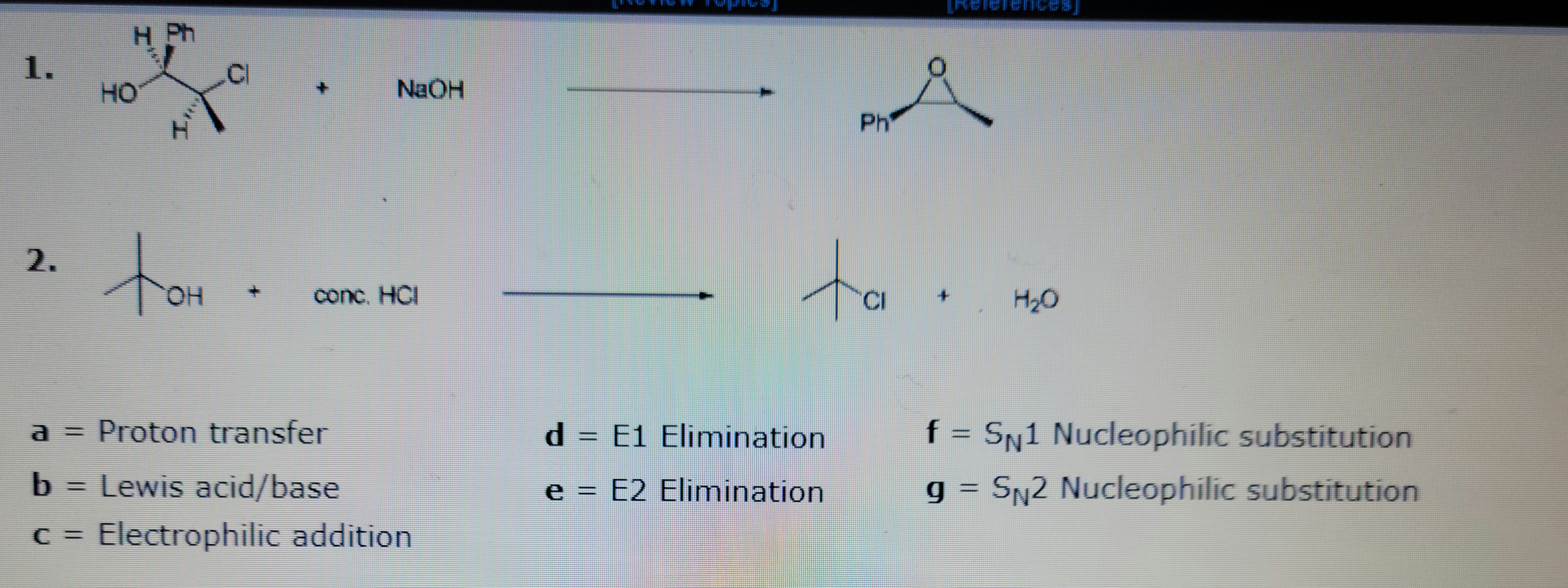
Transcribed Image Text:1.
2.
HO
11
OH
+
NaOH
conc. HCI
a = Proton transfer
b
Lewis acid/base
c = Electrophilic addition
d = E1
e = E2
Å
Elimination
Elimination
Ph
ta.
H₂O
f = SN1 Nucleophilic substitution
g = S₁2 Nucleophilic substitution
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution
Trending nowThis is a popular solution!
Step by stepSolved in 3 steps with 2 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- Ozone may decompose according to the following reaction mechanismI pictured below) a) Determine the rate law for the proposed mechanism assuming that oxygen radical is unstable and highly radicalarrow_forwardA possible mechanism for the gas phase reaction of NO and H2 is as follows: Step 1 2NO --k1-->N2O2 Step 2 N2O2 + H2 --k2--> N2O + H2O Step 3 N2O + H2 --k3--> N2 + H2O Which of the following statements concerning this mechanism is not supported by the information provided? a. Step 1 is the rate-determining step? b. N2O2 is an intermediate c. There is no catalyst in this reaction d. The rate expression for step 1 is rate = k[NO]2 e. All steps are bimolecular reactionsarrow_forwardThere are two factors associated with the Arrhenius Rate Constant Equation pre-exponential term, A. What are these factors and explain the meaning of each one?arrow_forward
- 13) Organic chemistry subject, please provide the correct solution for the following.arrow_forwardnd no Consider the following mechanism. (1) CIO'(aq) + H2O(I) =HC10(aq) + OH (aq) [fast] (2) I "(aq) + HCIO(aq) →HIO(aq) + Cl "(aq) [slow] (3) OH (aq) + HIO(aq) →H2O(1) + IO*(aq) [fast] (a) What is the overall equation? (b) Identify the intermediates, if any. (c) What is the molecularity and the rate law for each step?arrow_forwardwrite the mechanism OH 2.2 HO HO OH OCH, ง OH OH H*, H₂O + CH₂OH OH OHarrow_forward
- Under what condition can we use stochiometric equation to write rate lawarrow_forwardProblem: Given the reaction NO₂ (g) + CO (g) 2 (a) Determine the order with respect to 0₂ (b) Determine the order with respect to NO (c) Write the complete rate law (d) Determine the value of the rate constant k Experiment 1 2 3 Initial Rate (M/s) 0.00501 0.0803 0.00502 NO (g) + CO₂ (g) and initial rate data below: Initial [NO₂] (M) 0.100 0.400 0.100 Initial [CO] (M) 0.100 0.100 0.200arrow_forward
arrow_back_ios
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY