
General Chemistry - Standalone book (MindTap Course List)
11th Edition
ISBN: 9781305580343
Author: Steven D. Gammon, Ebbing, Darrell Ebbing, Steven D., Darrell; Gammon, Darrell Ebbing; Steven D. Gammon, Darrell D.; Gammon, Ebbing; Steven D. Gammon; Darrell
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Question
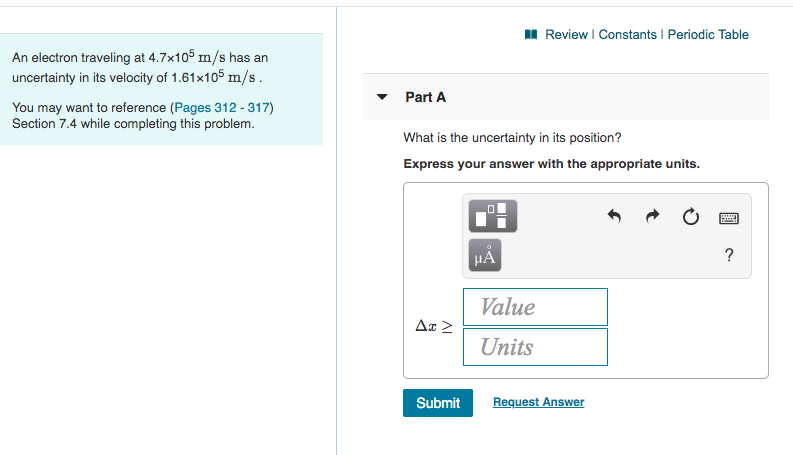
An electron traveling at 4.7×105 m/sm/s has an uncertainty in its velocity of 1.61×105 m/sm/s .
Transcribed Image Text:I Review I Constants I Periodic Table
An electron traveling at 4.7x105 m/s has an
uncertainty in its velocity of 1.61x105 m/s.
Part A
You may want to reference (Pages 312 - 317)
Section 7.4 while completing this problem.
What is the uncertainty in its position?
Express your answer with the appropriate units.
HẢ
Value
Units
Submit
Request Answer
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution
Trending nowThis is a popular solution!
Step by stepSolved in 2 steps with 1 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- 6.106 When Bohr devised his model for the atom, was he using deductive or inductive reasoning? Explain your answer.arrow_forwardThe energies of macroscopic objects, as well as those of microscopic objects, are quantized, but the effects of the quantization are not seen because the difference in energy between adjacent states is so small. Apply Bohr’s quantization of angular momentum to the revolution of Earth (mass6.01024kg) , which moves with a speed of 3.0104ms1 in a circular orbit (radius1.51011m) about the sun. The sun can be treated as fixed. Calculate the value of the quantum number n for the present state of the Earthsun system. What would be the effect of an increase in n by 1?arrow_forwardQuantum mechanics predicts that the energy of the ground state of the H atom is 13.6eV . Insight into the magnitude of this quantity is gained by considering several methods by which it can be measured. (a) Calculate the longest wavelength of light that will ionize H atoms in their ground state. (b) Assume the atom is ionized by collision with an electron that transfers all its kinetic energy to the atom in the ionization process. Calculate the speed of the electron before the collision. Express your answer in meters per second (ms1) and miles per hour (milesh1) . (c) Calculate the temperature required to ionize a H atom in its ground state by thermal excitation. (Hint: Recall the criterion for thermal excitation of an oscillator in Planck’s theory of blackbody radiation is that hvkBT .)arrow_forward
- How does probability fit into the description of the atom?arrow_forwardBohr described the hydrogen atom as an electron orbiting a hydrogen nucleus. Although certain aspects of his theory are still valid, his theory agreed quantitatively with experiment only in the case of the hydrogen atom. In what way does quantum mechanics change Bohrs original picture of the hydrogen atom?arrow_forwardState the postulates of quantum mechanics introduced throughout the chapter in your own words.arrow_forward
- Using Table 5.2, write down the mathematical expression for the 2px wave function for an electronically excited H atom. Estimate the probability of finding the 2px electron if you look in a cubical box of volume of 0.8(pm)3 centered at a distance of 0.5001010m in the =/2 , =0 direction. Does this probability change as you change ? At what angles is the probability of finding the electron smallest and at what angles is the probability the largest? (Note that =2 is the same location as =0 , so don’t double count.)arrow_forwardImagine a world in which the rule for the l quantum number is that values start with 1 and go up to n. The rules for the n and mi quantum numbers are unchanged from those of our world. Write the quantum numbers for the first two shells (i.e., n = 1 and n = 2).arrow_forwardUse the mathematical expression for the 2pz wave function of a one-electron atom (see Table 5.2) to show that the probability of finding an electron in that orbital anywhere in the x-y plane is 0. What are the nodal planes for a dxz orbital and for a dx2y2 orbital?arrow_forward
- Consider the orbitals shown here in outline. (a) What is the maximum number of electrons contained in an orbital of type (x)? Of type (y)? Of type (z)? (b) How many orbitals of type (x) are found in a shell with n=2? How many of type (y)? How many of type (z)? (c) Write a set of quantum numbers for an electron in an orbital of type (x) in a shell with n=4, of an orbital of type (y) in a shell with n=2. Of an orbital of type (z) in a shell with n=3. (d) What is the smallest possible n value for an orbital of type (x)? Of type (y)? Of type (z)? (e) What are the possible I and ml values for an orbital of type (x)? Of type (y)? Of type (z)?arrow_forwardThe wave function of an electron in the lowest (that is, ground) state of the hydrogen atom is (r)=( 1 a 0 3 )1/2exp(r a 0 )ao=0.5291010m (a) What is the probability of finding the electron inside a sphere of volume 1.0pm2 , centered at the nucleus (1pm=1012m) ? (b) What is the probability of finding the electron in a volume of 1.0pm2 at a distance of 52.9 pm from the nucleus, in a fixed but arbitrary direction? (c) What is the probability of finding the electron in a spherical shell of 1.0 pm in thickness, at a distance of 52.9 pm from the nucleus?arrow_forwardonsider the following statements: “The ionization energy for the potassium atom is negative because when K loses an electron to become K+ , it achieves a noble, gas. electron configuration." Indicate everything that is correct in this statement. Indicate everything that is incorrect. Correct the mistaken information and explain the error.arrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 General Chemistry - Standalone book (MindTap Cour...ChemistryISBN:9781305580343Author:Steven D. Gammon, Ebbing, Darrell Ebbing, Steven D., Darrell; Gammon, Darrell Ebbing; Steven D. Gammon, Darrell D.; Gammon, Ebbing; Steven D. Gammon; DarrellPublisher:Cengage Learning
General Chemistry - Standalone book (MindTap Cour...ChemistryISBN:9781305580343Author:Steven D. Gammon, Ebbing, Darrell Ebbing, Steven D., Darrell; Gammon, Darrell Ebbing; Steven D. Gammon, Darrell D.; Gammon, Ebbing; Steven D. Gammon; DarrellPublisher:Cengage Learning Introductory Chemistry: A FoundationChemistryISBN:9781337399425Author:Steven S. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
Introductory Chemistry: A FoundationChemistryISBN:9781337399425Author:Steven S. Zumdahl, Donald J. DeCostePublisher:Cengage Learning Chemistry for Engineering StudentsChemistryISBN:9781337398909Author:Lawrence S. Brown, Tom HolmePublisher:Cengage Learning
Chemistry for Engineering StudentsChemistryISBN:9781337398909Author:Lawrence S. Brown, Tom HolmePublisher:Cengage Learning ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning Chemistry: An Atoms First ApproachChemistryISBN:9781305079243Author:Steven S. Zumdahl, Susan A. ZumdahlPublisher:Cengage Learning
Chemistry: An Atoms First ApproachChemistryISBN:9781305079243Author:Steven S. Zumdahl, Susan A. ZumdahlPublisher:Cengage Learning

General Chemistry - Standalone book (MindTap Cour...
Chemistry
ISBN:9781305580343
Author:Steven D. Gammon, Ebbing, Darrell Ebbing, Steven D., Darrell; Gammon, Darrell Ebbing; Steven D. Gammon, Darrell D.; Gammon, Ebbing; Steven D. Gammon; Darrell
Publisher:Cengage Learning

Introductory Chemistry: A Foundation
Chemistry
ISBN:9781337399425
Author:Steven S. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry for Engineering Students
Chemistry
ISBN:9781337398909
Author:Lawrence S. Brown, Tom Holme
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry: An Atoms First Approach
Chemistry
ISBN:9781305079243
Author:Steven S. Zumdahl, Susan A. Zumdahl
Publisher:Cengage Learning
