
Human Anatomy & Physiology (11th Edition)
11th Edition
ISBN: 9780134580999
Author: Elaine N. Marieb, Katja N. Hoehn
Publisher: PEARSON
expand_more
expand_more
format_list_bulleted
Question
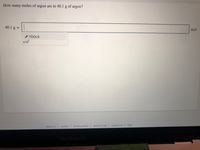
Transcribed Image Text:How many moles of argon are in 40.1 g of argon?
40.1 g =
%3D
mol
TOOLS
x10
terms of use contact us
| help
about us
careers
privacy policy
MacBook Air
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution
Trending nowThis is a popular solution!
Step by stepSolved in 2 steps

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, biology and related others by exploring similar questions and additional content below.Similar questions
- Balance the following (fictional) chemical equations by supplying the correct coefficient. Do not leave any space blank (in other words, write in 1 if you would be leaving it blank): R(OZ)2 - RY2+ Z20 a) ZY+ b) D2 (g) + L2 (g) – DL3 (e)arrow_forwardA microorganism was cultured in a flask containing a liquid medium. Samples were taken hourly and number of cells counted using a counting chamber. The data are provided below in cells per mL. Draw a growth curve in Excel and use it to calculate generation time. The answer must be provided as a number (in minutes). Time Cells per mL 81235849 0 6 7 10 26 11 12 13 14 15 16 17 18 19 20 21 22 23 24 25 2.00E+04 2.20E+04 2.30E+04 2.40E+04 3.10E+04 5.00E+04 8.06E+04 1.30E+05 2.09E+05 3.38E+05 5.44E+05 8.78E+05 1.41E+06 2.28E+06 3.68E+06 5.93E+06 9.56E+06 1.54E+07 2.48E+07 4.01E+07 6.46E+07 1.04E+08 1.68E+08 2.71E+08 2.72E+08 2.73E+08arrow_forwardThe kf of water is 1.86 kg °C/mol, approximately 4x the value of kp. If you attempted to freeze your 1.3m solution, how do you think the freezing point of water would be impacted?arrow_forward
- A compound contains only nitrogen and oxygen. Analysis of a/an 8.234 g sample of the compound reveals that the compound contains 4.118 grams of oxygen. How many grams of nitrogen does the sample contain? I need help with the formula for this question.arrow_forwardDetermine the number of moles in 1.95 x 1023 formula units of MgCl2.arrow_forwardHow many moles of HCl are there in 75.0 mL of 0.160 M HCl?arrow_forward
- 950 mL of 1X CuSO₄ * 5H₂O solution from 25X CuSO₄ * 5H₂O solutionarrow_forwardA sample of helium is initially at 605 torr in a volume of 2.85 L. At 24.7 °C you found the density of He to be 0.130 g/L and the density of Ar to be 1.30 g/L even though both samples had the same number of moles. Which one of the following best explains why the densities are different? A B C D Argon is a heavier atom than helium, so it has more mass in the same volume occupied by the gas. Argon is a larger atom than helium so it occupies more volume. An argon atom is more dense than a helium atom. The densities are different only because of rounding.arrow_forwardIf I put 80g of NaOH in 0.5L of H2O, what is the molar out of solution?arrow_forward
- According to Aristotle’s density equation, if 4.686 grams of the sugar glucose (C6H12O6) occupies a volume of 3.0 cubic centimeters, then glucose must have a density of: 14.058 grams/cm3 7.686 grams/cm3 1.686 grams/cm3 1.562 grams/cm3 0.6402 grams/cm3arrow_forwardoriginal molarity= .548 A 5.00 mL sample of this KOH solution is added to a 100 mL volumetric flask, and water is added to the mark. What is the new molarity of K+ ions in this solution?arrow_forwardAt 25.0 °C the Henry's Law constant for dinitrogen monoxide (N,0) gas in water is 0.025 M/atm. Calculate the mass in grams of N,0 gas that can be dissolved in 450. mL of water at 25.0 °C and a N,0 partial pressure of 1.31 atm. Be sure your answer has the correct number of significant digits. ?arrow_forward
arrow_back_ios
arrow_forward_ios
Recommended textbooks for you
 Human Anatomy & Physiology (11th Edition)BiologyISBN:9780134580999Author:Elaine N. Marieb, Katja N. HoehnPublisher:PEARSON
Human Anatomy & Physiology (11th Edition)BiologyISBN:9780134580999Author:Elaine N. Marieb, Katja N. HoehnPublisher:PEARSON Biology 2eBiologyISBN:9781947172517Author:Matthew Douglas, Jung Choi, Mary Ann ClarkPublisher:OpenStax
Biology 2eBiologyISBN:9781947172517Author:Matthew Douglas, Jung Choi, Mary Ann ClarkPublisher:OpenStax Anatomy & PhysiologyBiologyISBN:9781259398629Author:McKinley, Michael P., O'loughlin, Valerie Dean, Bidle, Theresa StouterPublisher:Mcgraw Hill Education,
Anatomy & PhysiologyBiologyISBN:9781259398629Author:McKinley, Michael P., O'loughlin, Valerie Dean, Bidle, Theresa StouterPublisher:Mcgraw Hill Education, Molecular Biology of the Cell (Sixth Edition)BiologyISBN:9780815344322Author:Bruce Alberts, Alexander D. Johnson, Julian Lewis, David Morgan, Martin Raff, Keith Roberts, Peter WalterPublisher:W. W. Norton & Company
Molecular Biology of the Cell (Sixth Edition)BiologyISBN:9780815344322Author:Bruce Alberts, Alexander D. Johnson, Julian Lewis, David Morgan, Martin Raff, Keith Roberts, Peter WalterPublisher:W. W. Norton & Company Laboratory Manual For Human Anatomy & PhysiologyBiologyISBN:9781260159363Author:Martin, Terry R., Prentice-craver, CynthiaPublisher:McGraw-Hill Publishing Co.
Laboratory Manual For Human Anatomy & PhysiologyBiologyISBN:9781260159363Author:Martin, Terry R., Prentice-craver, CynthiaPublisher:McGraw-Hill Publishing Co. Inquiry Into Life (16th Edition)BiologyISBN:9781260231700Author:Sylvia S. Mader, Michael WindelspechtPublisher:McGraw Hill Education
Inquiry Into Life (16th Edition)BiologyISBN:9781260231700Author:Sylvia S. Mader, Michael WindelspechtPublisher:McGraw Hill Education

Human Anatomy & Physiology (11th Edition)
Biology
ISBN:9780134580999
Author:Elaine N. Marieb, Katja N. Hoehn
Publisher:PEARSON

Biology 2e
Biology
ISBN:9781947172517
Author:Matthew Douglas, Jung Choi, Mary Ann Clark
Publisher:OpenStax

Anatomy & Physiology
Biology
ISBN:9781259398629
Author:McKinley, Michael P., O'loughlin, Valerie Dean, Bidle, Theresa Stouter
Publisher:Mcgraw Hill Education,

Molecular Biology of the Cell (Sixth Edition)
Biology
ISBN:9780815344322
Author:Bruce Alberts, Alexander D. Johnson, Julian Lewis, David Morgan, Martin Raff, Keith Roberts, Peter Walter
Publisher:W. W. Norton & Company

Laboratory Manual For Human Anatomy & Physiology
Biology
ISBN:9781260159363
Author:Martin, Terry R., Prentice-craver, Cynthia
Publisher:McGraw-Hill Publishing Co.

Inquiry Into Life (16th Edition)
Biology
ISBN:9781260231700
Author:Sylvia S. Mader, Michael Windelspecht
Publisher:McGraw Hill Education